Alkali metals elements
Alkali Metals Elements. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. They are placed in the vertical column on the left hand side of the periodic table. The alkali metals are shiny soft highly reactive metals at standard temperature and pressure. These do not occur in the native state i e do not occur free in nature.
 Characteristics Of The Compounds Of The Alkali Metals Oxides Videos Etc From toppr.com
Characteristics Of The Compounds Of The Alkali Metals Oxides Videos Etc From toppr.com
They are placed in the vertical column on the left hand side of the periodic table. The alkali metals are the elements located in group ia of the periodic table. The alkali metals are lithium sodium potassium rubidium cesium and francium. The alkali metals are shiny soft highly reactive metals at standard temperature and pressure. Sodium and potassium were discovered by davy rubidium and caesium by bunsen and kirchhoff while francium by perey. Sodium na potassium k rubidium rb cesium cs francium fr they re part of the s block of elements in the periodic table that along with hydrogen helium calcium and others have their outermost electron in an s orbital.
Sodium s high abundance is due mainly to large underground deposits of rock salt nacl.
The alkali metals found in group 1 of the periodic table formally known as group ia are so reactive that they are generally found in nature combined with other elements. These do not occur in the native state i e do not occur free in nature. The alkali metals are the elements located in group ia of the periodic table. However sodium is also more abundant in seawater 10 800 ppm as compared to potassium 380 ppm. The alkali metals found in group 1 of the periodic table formally known as group ia are so reactive that they are generally found in nature combined with other elements. They are placed in the vertical column on the left hand side of the periodic table.
 Source: emedicalprep.com
Source: emedicalprep.com
The elements of group 1a are known as alkali metals because they react with the water forming alkaline solutions. Lithium is known as a bridge element and was discovered by arfwedson. The elements of group 1a are known as alkali metals because they react with the water forming alkaline solutions. The alkali metals found in group 1 of the periodic table formally known as group ia are so reactive that they are generally found in nature combined with other elements. Group 1 of the periodic table of elements consists of hydrogen and below it the six alkali metals.
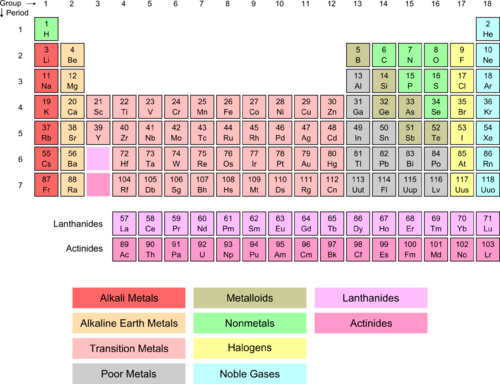 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The group 1 elements are called the alkali metals. Group 1 of the periodic table of elements consists of hydrogen and below it the six alkali metals. These elements are known as alkali metals. Sodium s high abundance is due mainly to large underground deposits of rock salt nacl. The elements of group 1a are known as alkali metals because they react with the water forming alkaline solutions.
 Source: toppr.com
Source: toppr.com
Lithium sodium potassium rubidium cesium and francium. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. These elements are known as alkali metals. The alkali metals found in group 1 of the periodic table formally known as group ia are so reactive that they are generally found in nature combined with other elements. Sodium and potassium were discovered by davy rubidium and caesium by bunsen and kirchhoff while francium by perey.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The alkali metals are the elements located in group ia of the periodic table. The alkali metals are the elements located in group ia of the periodic table. The alkali metals are lithium sodium potassium rubidium cesium and francium. The alkali metals are lithium sodium potassium rubidium cesium and francium. Sodium na potassium k rubidium rb cesium cs francium fr they re part of the s block of elements in the periodic table that along with hydrogen helium calcium and others have their outermost electron in an s orbital.
 Source: emedicalprep.com
Source: emedicalprep.com
The alkali metals are lithium sodium potassium rubidium cesium and francium. The group 1 elements are called the alkali metals. In the periodic table the alkali metals are a group or column containing the chemical elements such as lithium li sodium na rubidium rb potassium k francium fr and caesium cs. The alkali metals found in group 1 of the periodic table formally known as group ia are so reactive that they are generally found in nature combined with other elements. Sodium and potassium were discovered by davy rubidium and caesium by bunsen and kirchhoff while francium by perey.
 Source: brainly.com
Source: brainly.com
The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. Lithium is known as a bridge element and was discovered by arfwedson. The alkali metals are lithium sodium potassium rubidium cesium and francium. These do not occur in the native state i e do not occur free in nature. These elements are known as alkali metals.
 Source: breakingatom.com
Source: breakingatom.com
Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Sodium s high abundance is due mainly to large underground deposits of rock salt nacl. The alkali metals are the elements located in group ia of the periodic table. The alkali metals are shiny soft highly reactive metals at standard temperature and pressure. They are placed in the vertical column on the left hand side of the periodic table.
 Source: britannica.com
Source: britannica.com
The alkali metals are lithium sodium potassium rubidium cesium and francium. The alkali metals are the elements located in group ia of the periodic table. Sodium na potassium k rubidium rb cesium cs francium fr they re part of the s block of elements in the periodic table that along with hydrogen helium calcium and others have their outermost electron in an s orbital. They are placed in the vertical column on the left hand side of the periodic table. The alkali metals are lithium sodium potassium rubidium cesium and francium.
 Source: online.seterra.com
Source: online.seterra.com
The alkali metals are the elements located in group ia of the periodic table. The alkali metals are soft metals that are highly reactive with water and oxygen. Group 1 of the periodic table of elements consists of hydrogen and below it the six alkali metals. All the group 1 elements are very reactive. Sodium and potassium were discovered by davy rubidium and caesium by bunsen and kirchhoff while francium by perey.
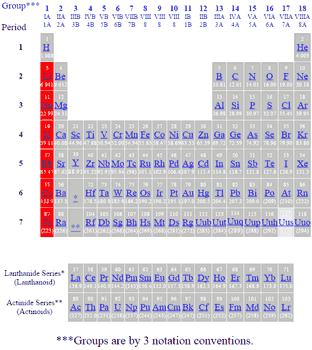 Source: gordonengland.co.uk
Source: gordonengland.co.uk
The alkali metals are lithium sodium potassium rubidium cesium and francium. Sodium s high abundance is due mainly to large underground deposits of rock salt nacl. Potassium is more active than sodium because the atomic size of potassium is larger than of sodium. The group 1 elements are called the alkali metals. Alkali metals are monovalent elements because they tend to lose the valency electron during the chemical reaction.
 Source: toppr.com
Source: toppr.com
They are placed in the vertical column on the left hand side of the periodic table. The alkali metals are soft metals that are highly reactive with water and oxygen. The abundance of the alkali metals is given in table 3 1. Sodium na potassium k rubidium rb cesium cs francium fr they re part of the s block of elements in the periodic table that along with hydrogen helium calcium and others have their outermost electron in an s orbital. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table.
 Source: chem4kids.com
Source: chem4kids.com
All the group 1 elements are very reactive. These do not occur in the native state i e do not occur free in nature. The abundance of the alkali metals is given in table 3 1. The alkali metals are lithium sodium potassium rubidium cesium and francium. They are placed in the vertical column on the left hand side of the periodic table.

The alkali metals are lithium sodium potassium rubidium cesium and francium. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Alkali metals are monovalent elements because they tend to lose the valency electron during the chemical reaction. Lithium is known as a bridge element and was discovered by arfwedson. In the periodic table the alkali metals are a group or column containing the chemical elements such as lithium li sodium na rubidium rb potassium k francium fr and caesium cs.
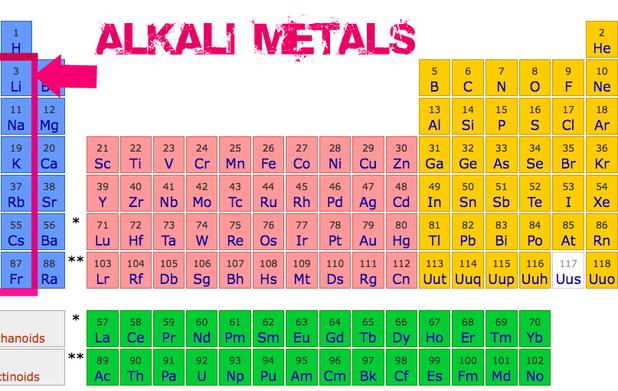 Source: online-sciences.com
Source: online-sciences.com
All the group 1 elements are very reactive. The group 1 elements are called the alkali metals. Sodium na potassium k rubidium rb cesium cs francium fr they re part of the s block of elements in the periodic table that along with hydrogen helium calcium and others have their outermost electron in an s orbital. The alkali metals are soft metals that are highly reactive with water and oxygen. These do not occur in the native state i e do not occur free in nature.

The group 1 elements are called the alkali metals. The alkali metals are lithium sodium potassium rubidium cesium and francium. Potassium is more active than sodium because the atomic size of potassium is larger than of sodium. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Lithium sodium potassium rubidium cesium and francium.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alkali metals elements by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





