Alkaline earth metals examples
Alkaline Earth Metals Examples. Structurally they together with helium have in common an outer s. Ca cl 2 cacl 2. They form 1 ions while the alkaline earth metals form 2 ions. Beryllium radium calcium magnesium strontium barrium.
 Alkali Metals And Alkaline Earth Metals By Aurora Sutori From sutori.com
Alkali Metals And Alkaline Earth Metals By Aurora Sutori From sutori.com
Examples of alkaline earth metals include calcium which is needed for strong bones and strontium which is used for making cement and other products. Ba i 2 bai 2. While most metals are hard lead and gallium are examples of elements that are soft. This reaction is a exothermic reaction. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. Ca cl 2 cacl 2.
Examples of alkaline earth metals.
Examples of alkaline earth metals. Alkaline earth metals react with halogens and form halides. Structurally they together with helium have in common an outer s. This reaction is a exothermic reaction. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Ammonia and alkaline earth metals.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The alkali metals are generally more reactive than the alkaline earth metals. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. These elements tend to have lower melting and boiling points than the transition metals with some exceptions. They form 1 ions while the alkaline earth metals form 2 ions. Examples of alkaline earth metals include calcium which is needed for strong bones and strontium which is used for making cement and other products.
 Source: emedicalprep.com
Source: emedicalprep.com
Structurally they together with helium have in common an outer s. Explore more at the following url observe how four different alkaline earth metals react with water. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Alkaline earth metals react with halogens and form halides. They form 1 ions while the alkaline earth metals form 2 ions.
 Source: pinterest.com
Source: pinterest.com
They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. These elements tend to have lower melting and boiling points than the transition metals with some exceptions. Examples of alkaline earth metals. Sulfur and alkaline earth metals reactions. Alkaline earth metal any of the six chemical elements that comprise group 2 iia of the periodic table.
 Source: chem4kids.com
Source: chem4kids.com
Beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. Due to release of hydrogen gas ammonia behaves as an acid. Mgs hcl mgcl 2 h 2 s. All the alkaline earths except for beryllium form corrosive alkaline hydroxides. The elements are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
 Source: isbchem1.pbworks.com
Source: isbchem1.pbworks.com
This metal sulfide reacts with dilute acids and emit hydrogen sulfide gas. Ammonia and alkaline earth metals. Examples of alkaline earth metals include calcium which is needed for strong bones and strontium which is used for making cement and other products. Structurally they together with helium have in common an outer s. The alkali metals are generally more reactive than the alkaline earth metals.
 Source: youtube.com
Source: youtube.com
While most metals are hard lead and gallium are examples of elements that are soft. This reaction is a exothermic reaction. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Due to release of hydrogen gas ammonia behaves as an acid. Sulfur and alkaline earth metals reactions.
Source: saddlespace.org
Explore more at the following url observe how four different alkaline earth metals react with water. This reaction is a exothermic reaction. Beryllium radium calcium magnesium strontium barrium. They form 1 ions while the alkaline earth metals form 2 ions. The alkali metals are generally more reactive than the alkaline earth metals.
 Source: sutori.com
Source: sutori.com
Ammonia and alkaline earth metals. 3mg 2nh 3 mg 3 n 2 h 2. The alkali metals are generally more reactive than the alkaline earth metals. While most metals are hard lead and gallium are examples of elements that are soft. Elemental magnesium is the only alkaline earth metal that is produced on a large scale about 5 10 5 tn per year.
 Source: toppr.com
Source: toppr.com
The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. While most metals are hard lead and gallium are examples of elements that are soft. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. The alkali metals are generally more reactive than the alkaline earth metals. Ammonia and alkaline earth metals.
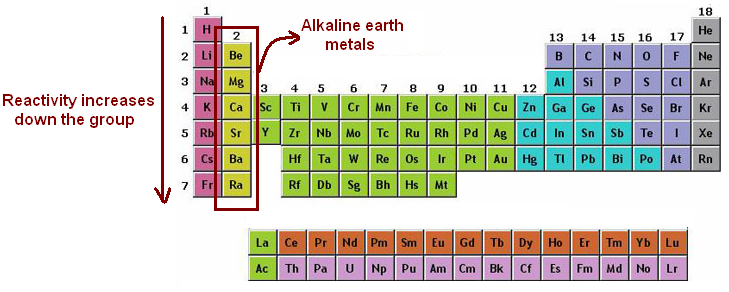 Source: makaylasharanscience2ndperiod.weebly.com
Source: makaylasharanscience2ndperiod.weebly.com
Alkaline earth metal any of the six chemical elements that comprise group 2 iia of the periodic table. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Ammonia and alkaline earth metals. Elemental magnesium is the only alkaline earth metal that is produced on a large scale about 5 10 5 tn per year. Beryllium radium calcium magnesium strontium barrium.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Explore more at the following url observe how four different alkaline earth metals react with water. This metal sulfide reacts with dilute acids and emit hydrogen sulfide gas. Alkaline earth metal any of the six chemical elements that comprise group 2 iia of the periodic table. Alkali metal compounds tend to be more soluble in. Beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
 Source: shmoop.com
Source: shmoop.com
Explore more at the following url observe how four different alkaline earth metals react with water. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Ba i 2 bai 2. The alkali metals are generally more reactive than the alkaline earth metals. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
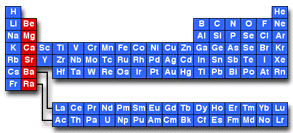 Source: ilpi.com
Source: ilpi.com
Beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. They form 1 ions while the alkaline earth metals form 2 ions. All of the alkaline earths react with halogens to form halides. Elemental magnesium is the only alkaline earth metal that is produced on a large scale about 5 10 5 tn per year. Explore more at the following url observe how four different alkaline earth metals react with water.
 Source: chemistrylearner.com
Source: chemistrylearner.com
While most metals are hard lead and gallium are examples of elements that are soft. While most metals are hard lead and gallium are examples of elements that are soft. Explore more at the following url observe how four different alkaline earth metals react with water. The alkali metals are generally more reactive than the alkaline earth metals. Beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
 Source: toppr.com
Source: toppr.com
Alkaline earth metals react with halogens and form halides. Due to release of hydrogen gas ammonia behaves as an acid. Ammonia and alkaline earth metals. This metal sulfide reacts with dilute acids and emit hydrogen sulfide gas. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alkaline earth metals examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






