Amylose iodine test
Amylose Iodine Test. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color.
 Low Density Graphitic Films Prepared From Iodine Doped Enzymatically Synthesized Amylose Films As Carbonization Precursors Sciencedirect From sciencedirect.com
Low Density Graphitic Films Prepared From Iodine Doped Enzymatically Synthesized Amylose Films As Carbonization Precursors Sciencedirect From sciencedirect.com
This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. It is also used by chemists testing for or titrating iodine. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. Iodine ki reagent. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. The amylose iodine complex rotatable in 3 dimensions.
Chemical test for starch or iodine.
As starch is nothing but carbohydrates the test for carbohydrates is same as that of starch. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. The display and interactive features of this page cannot be accessed if javascript is not enabled. 3 iodine test the amylose unbranched linear portion of starch forms helices which allow iodine molecules to assemble forming a dark blue black color refer to diagram below. Iodine atoms can then fit into the helices to form a starch iodine or glycogen iodine complex. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color.
 Source: sciencedirect.com
Source: sciencedirect.com
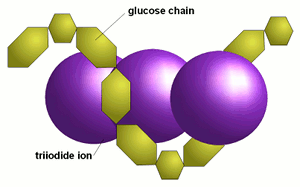
3 iodine test the amylose unbranched linear portion of starch forms helices which allow iodine molecules to assemble forming a dark blue black color refer to diagram below. Iodine is used as a test for starch both linear amylose and branched amylopectin because of the distinct colour change deep orange to dark blue that occurs when it forms a polyiodide complex with certain polysaccharides. The amylose iodine complex rotatable in 3 dimensions. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. Iodine atoms can then fit into the helices to form a starch iodine or glycogen iodine complex.
 Source: smallscalechemistry.colostate.edu
Source: smallscalechemistry.colostate.edu
The starch iodine test is a standard test for starch often used by biologists and biochemists. Effect of temperature on digestion of starch by amylase benedict test reducing sugars test detecting the presence of glucose experiment with salivary amylase enzyme this video shows a demonstration of the enzyme salivary amylase which is present in our saliva. The enzyme aids in digestion by breaking down starch molecules present. The starch iodine test is a standard test for starch often used by biologists and biochemists. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance.
 Source: researchgate.net
Source: researchgate.net
Iodine is used as a test for starch both linear amylose and branched amylopectin because of the distinct colour change deep orange to dark blue that occurs when it forms a polyiodide complex with certain polysaccharides. The iodine molecule slips inside of the amylose coil. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color. Iodine test is a chemical test used to distinguish mono or disaccharides from certain polysaccharides like amylase dextrin and glycogen. Iodine ki reagent.
Source:
This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. One of the most important iodine test is that for detecting thyroid problems. Iodine atoms can then fit into the helices to form a starch iodine or glycogen iodine complex. The starch iodine test is a standard test for starch often used by biologists and biochemists.
 Source: braukaiser.com
Source: braukaiser.com
3 iodine test the amylose unbranched linear portion of starch forms helices which allow iodine molecules to assemble forming a dark blue black color refer to diagram below. Chemical test for starch or iodine. Using iodine to test for the presence of starch is a common experiment. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. The iodine molecule slips inside of the amylose coil.
 Source: researchgate.net
Source: researchgate.net
Chemical test for starch or iodine. The display and interactive features of this page cannot be accessed if javascript is not enabled. Using iodine to test for the presence of starch is a common experiment. Effect of temperature on digestion of starch by amylase benedict test reducing sugars test detecting the presence of glucose experiment with salivary amylase enzyme this video shows a demonstration of the enzyme salivary amylase which is present in our saliva. Abstract the absorbance pattern of amylose iodine complex from cassava starch sample was examined by scanning of uv visible spectra between 200 800 nm.
 Source: chemistryviews.org
Source: chemistryviews.org
Iodine test is a chemical test used to distinguish mono or disaccharides from certain polysaccharides like amylase dextrin and glycogen. The display and interactive features of this page cannot be accessed if javascript is not enabled. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. Iodine atoms can then fit into the helices to form a starch iodine or glycogen iodine complex.
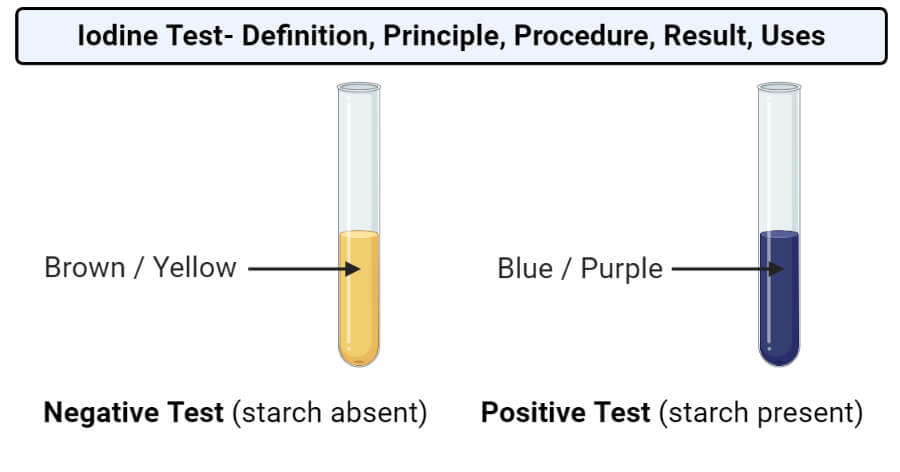 Source: microbenotes.com
Source: microbenotes.com
Effect of temperature on digestion of starch by amylase benedict test reducing sugars test detecting the presence of glucose experiment with salivary amylase enzyme this video shows a demonstration of the enzyme salivary amylase which is present in our saliva. The amylose iodine complex rotatable in 3 dimensions. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. 3 iodine test the amylose unbranched linear portion of starch forms helices which allow iodine molecules to assemble forming a dark blue black color refer to diagram below. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. Chemical test for starch or iodine. As starch is nothing but carbohydrates the test for carbohydrates is same as that of starch. Abstract the absorbance pattern of amylose iodine complex from cassava starch sample was examined by scanning of uv visible spectra between 200 800 nm. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue.
 Source: sciencedirect.com
Source: sciencedirect.com
Iodine is used as a test for starch both linear amylose and branched amylopectin because of the distinct colour change deep orange to dark blue that occurs when it forms a polyiodide complex with certain polysaccharides. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. Chemical test for starch or iodine. Iodine ki reagent.
 Source: byjus.com
Source: byjus.com
As starch is nothing but carbohydrates the test for carbohydrates is same as that of starch. The iodine molecule slips inside of the amylose coil. The display and interactive features of this page cannot be accessed if javascript is not enabled. Chemical test for starch or iodine. Starch in the form of amylose and amylopectin has less branches than glycogen.
 Source: degruyter.com
Source: degruyter.com
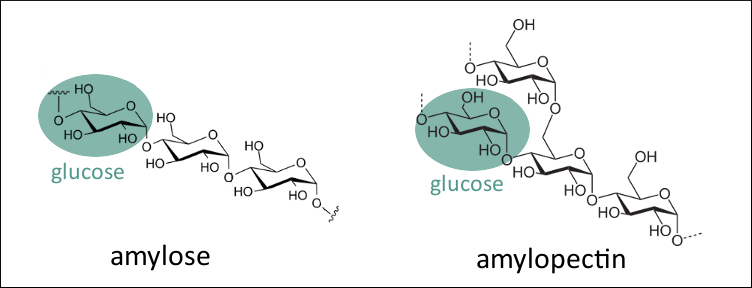
The enzyme aids in digestion by breaking down starch molecules present. This means that the helices of starch are longer than glycogen therefore binding more iodine atoms. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves. The amylopectin branched portion of starch forms much shorter helices due to the branching present and iodine molecules are unable to assemble leading the color to be of red brown or red violet.
 Source: chemistryviews.org
Source: chemistryviews.org
As starch is nothing but carbohydrates the test for carbohydrates is same as that of starch. Iodine atoms can then fit into the helices to form a starch iodine or glycogen iodine complex. The display and interactive features of this page cannot be accessed if javascript is not enabled. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide.
 Source: tangofscience.blog
Source: tangofscience.blog
Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. In the presence of iodine amylose in starch forms a deep blue color. Starch in the form of amylose and amylopectin has less branches than glycogen. Iodine is used as a test for starch both linear amylose and branched amylopectin because of the distinct colour change deep orange to dark blue that occurs when it forms a polyiodide complex with certain polysaccharides.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
Abstract the absorbance pattern of amylose iodine complex from cassava starch sample was examined by scanning of uv visible spectra between 200 800 nm. Iodine ki reagent. As starch is nothing but carbohydrates the test for carbohydrates is same as that of starch. Starch in the form of amylose and amylopectin has less branches than glycogen. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title amylose iodine test by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.