Characteristic of periodic table
Characteristic Of Periodic Table. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. When the chemical elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. The table is the arrangement of elements in increasing order of their atomic numbers.
 Family Characteristics And Properties The Periodic Table Of Elements From 170188733453308075.weebly.com
Family Characteristics And Properties The Periodic Table Of Elements From 170188733453308075.weebly.com
Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus. The horizontal rows of elements in periodic table are called periods. The characteristics of modern periodic table are. The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7. It consists of 18 vertical columns and 7 horizontal rows. The modern or long form of the periodic table is based on the modern periodic law.
The horizontal rows of elements in periodic table are called periods.
The table is the arrangement of elements in increasing order of their atomic numbers. The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus. The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. The modern or long form of the periodic table is based on the modern periodic law.
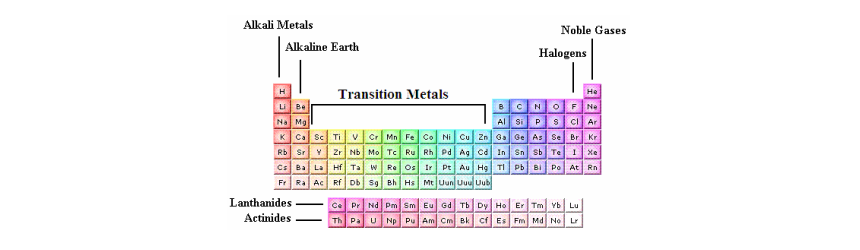
 Source: onlinesciencenotes.com
Source: onlinesciencenotes.com
The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7. Characteristics of mendeleev s periodic table. The block of transitional metals which is present in middle part of the modern periodic table includes elements of group ib to viiib i e. The horizontal rows of elements in periodic table are called periods. 3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements.
 Source: britannica.com
Source: britannica.com
The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. 3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements. It consists of 18 vertical columns and 7 horizontal rows. There are 7 periods in modern periodic table.
 Source: britannica.com
Source: britannica.com
Why arrange elements in a table. The table is the arrangement of elements in increasing order of their atomic numbers. The arrangement of elements in modern periodic table is based on their electronic configurations. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus. The characteristics of modern periodic table are.
 Source: quora.com
Source: quora.com
The modern periodic table is the present form of the periodic table. The characteristics of modern periodic table are. The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7. The modern periodic table is the present form of the periodic table. Characteristics of mendeleev s periodic table.
 Source: pinterest.com
Source: pinterest.com
The modern or long form of the periodic table is based on the modern periodic law. In mendeleev periodic table vertical columns in the periodic table and horizontal row in the periodic table were named as groups and period respectively. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus. The arrangement of elements in modern periodic table is based on their electronic configurations. Why arrange elements in a table.
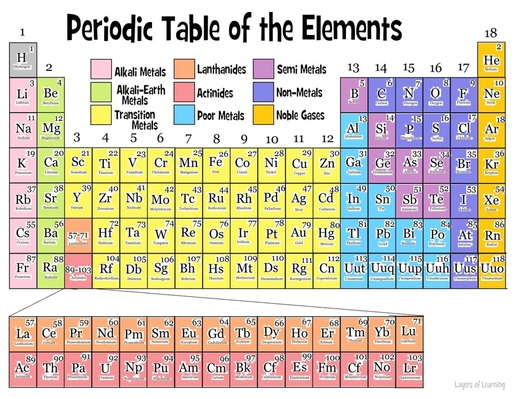 Source: periodic-table-components.weebly.com
Source: periodic-table-components.weebly.com
The block of transitional metals which is present in middle part of the modern periodic table includes elements of group ib to viiib i e. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the. Characteristics of mendeleev s periodic table. It consists of 18 vertical columns and 7 horizontal rows.
 Source: kullabs.com
Source: kullabs.com
The modern periodic table is the present form of the periodic table. Characteristics of mendeleev s periodic table. The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups. The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7. The modern or long form of the periodic table is based on the modern periodic law.
 Source: frontierchemists.blogspot.com
Source: frontierchemists.blogspot.com
The arrangement of elements in modern periodic table is based on their electronic configurations. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the. 3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements. The arrangement of elements in modern periodic table is based on their electronic configurations.
 Source: kullabs.com
Source: kullabs.com
The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. The characteristics of modern periodic table are. When the chemical elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The modern periodic table is the present form of the periodic table. It consists of 18 vertical columns and 7 horizontal rows.
 Source: slideshare.net
Source: slideshare.net
The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. When the chemical elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. The modern or long form of the periodic table is based on the modern periodic law. The modern periodic table is the present form of the periodic table.
 Source: prezi.com
Source: prezi.com
When the chemical elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. The block of transitional metals which is present in middle part of the modern periodic table includes elements of group ib to viiib i e. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number i e the total number of protons in the atomic nucleus.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The arrangement of elements in modern periodic table is based on their electronic configurations. Characteristics of mendeleev s periodic table. 3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. The block of transitional metals which is present in middle part of the modern periodic table includes elements of group ib to viiib i e.
 Source: researchgate.net
Source: researchgate.net
3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements. The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups. The characteristics of modern periodic table are. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. The periodic table is composed of seven horizontal rows or periods and is numbered between 1 and 7.
 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
The characteristics of modern periodic table are. The modern periodic table is the present form of the periodic table. The horizontal rows of elements in periodic table are called periods. 3 to 12 these elements have last electron in d subshell their properties are midway between those of s block p block so they are called transitional elements. The arrangement of elements in modern periodic table is based on their electronic configurations.
 Source: studylib.net
Source: studylib.net
The table is the arrangement of elements in increasing order of their atomic numbers. There are 7 periods in modern periodic table. The modern periodic table is the present form of the periodic table. The table is the arrangement of elements in increasing order of their atomic numbers. The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title characteristic of periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.