Charles boyles law
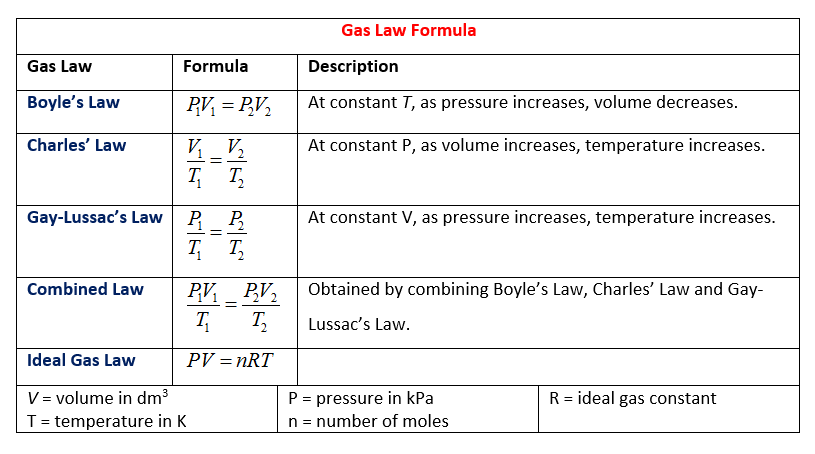
Charles Boyles Law. In this article we are going to discuss what charles law and boyle s law are their definitions applications of charles law and boyle s law their similarities and finally the differences between charles law and boyle s law. Boyle s law charles s law and gay lussac s law form the combined gas law. Therefore the volume of container 1 is 20 l. Boyle s law is a gas law.
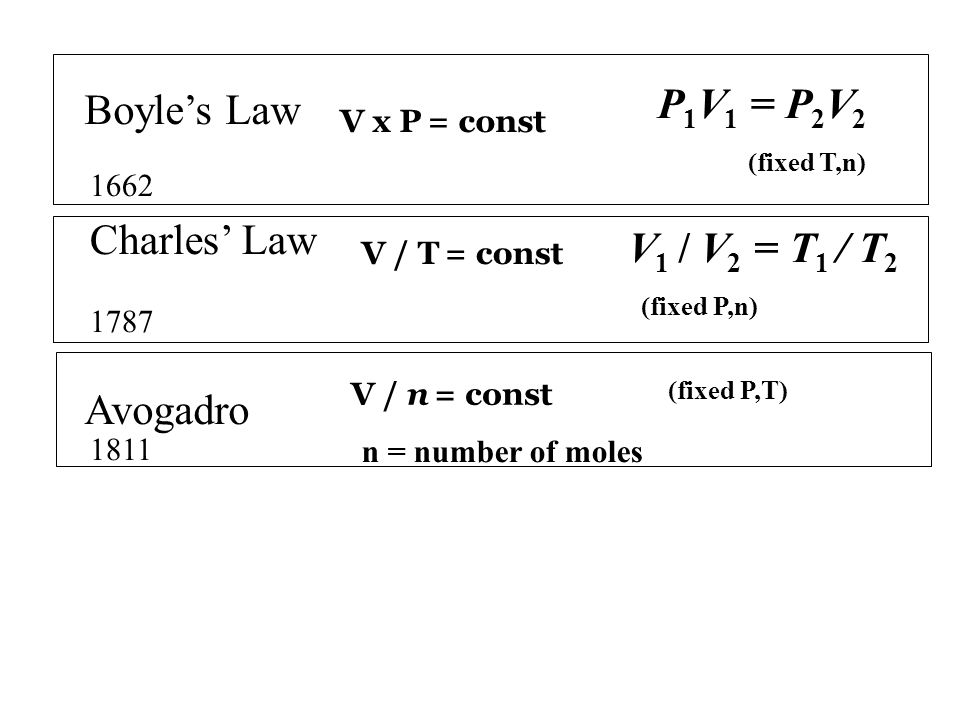
Boyle s law is a gas law. This equation called boyle s law says that all other factors being the same the product of pressure and volume pv will be conserved. Ideal gas law makes use of a theoretical gas that has the following properties. In this article we are going to discuss what charles law and boyle s law are their definitions applications of charles law and boyle s law their similarities and finally the differences between charles law and boyle s law. The aim is simply to show how these laws relate to kinetic theory in a non mathematical way and to the ideal gas equation. Boyle s law is often used as part of an explanation on how the breathing system works in the human body.
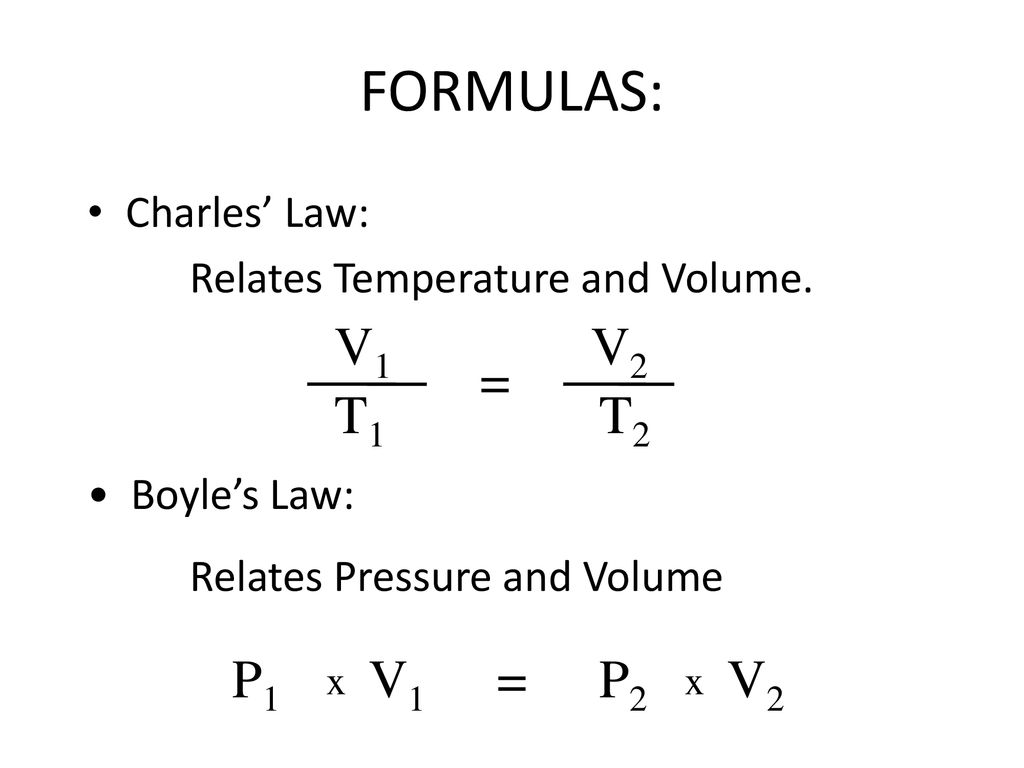
From boyle s law and charles law charles law is better for the purpose of thermometry with increasing temperature pressure and volume of the gas also increases.
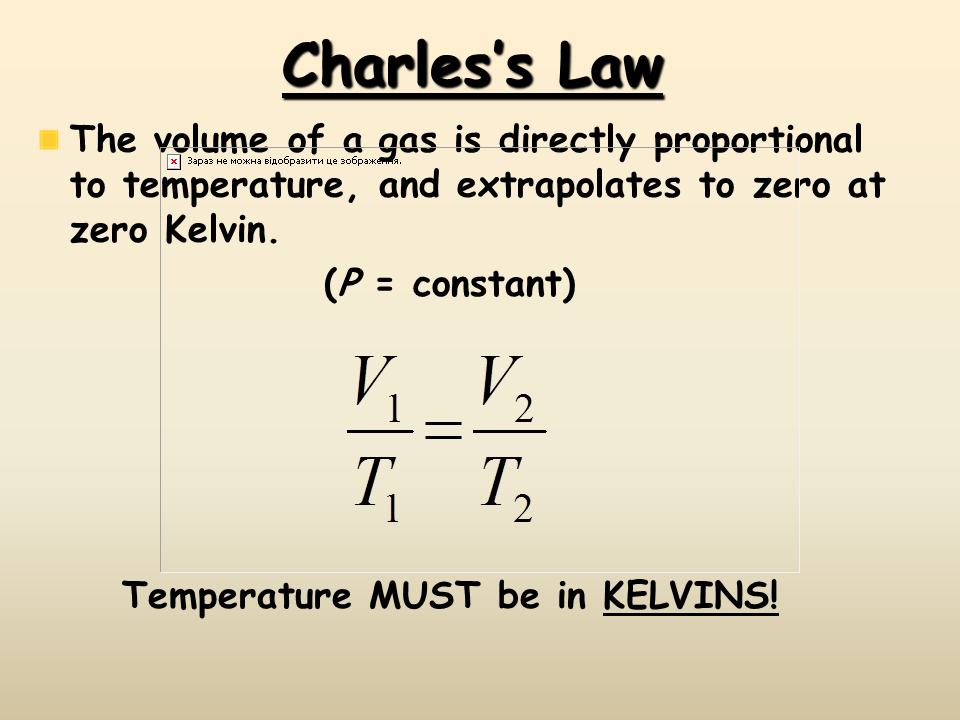
At constant pressure volume is proportional to absolute temperature. At constant pressure you can say that the following relationship is true for an ideal gas. This page takes a simple look at boyle s law and charles law and is suitable for 16 18 year old chemistry students doing a course the equivalent of uk a level. Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume. Therefore the volume of container 1 is 20 l. To learn more about boyle s law and other important gas laws such as charles law register with byju s and download the mobile application on your smartphone.
 Source: pinterest.com
Source: pinterest.com
This experiment is carried out by inserting a certain amount of gas into a closed container. In this article we are going to discuss what charles law and boyle s law are their definitions applications of charles law and boyle s law their similarities and finally the differences between charles law and boyle s law. Ideal gas law makes use of a theoretical gas that has the following properties. Boyle s law charles s law and gay lussac s law form the combined gas law. Therefore the volume of container 1 is 20 l.
 Source: pinterest.com
Source: pinterest.com
Therefore the volume of container 1 is 20 l. At constant pressure volume is proportional to absolute temperature. Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume. Boyle s law charles s law gay lussac s law. This commonly involves explaining how the lung.

At constant pressure you can say that the following relationship is true for an ideal gas. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. Boyle s law charles s law and gay lussac s law form the combined gas law. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to. At constant pressure volume is proportional to absolute temperature.
 Source: pdffiller.com
Source: pdffiller.com
To learn more about boyle s law and other important gas laws such as charles law register with byju s and download the mobile application on your smartphone. Boyle s law is a gas law. It is defined for an ideal gas. P f v f p i v i. This page takes a simple look at boyle s law and charles law and is suitable for 16 18 year old chemistry students doing a course the equivalent of uk a level.
 Source: slideplayer.com
Source: slideplayer.com
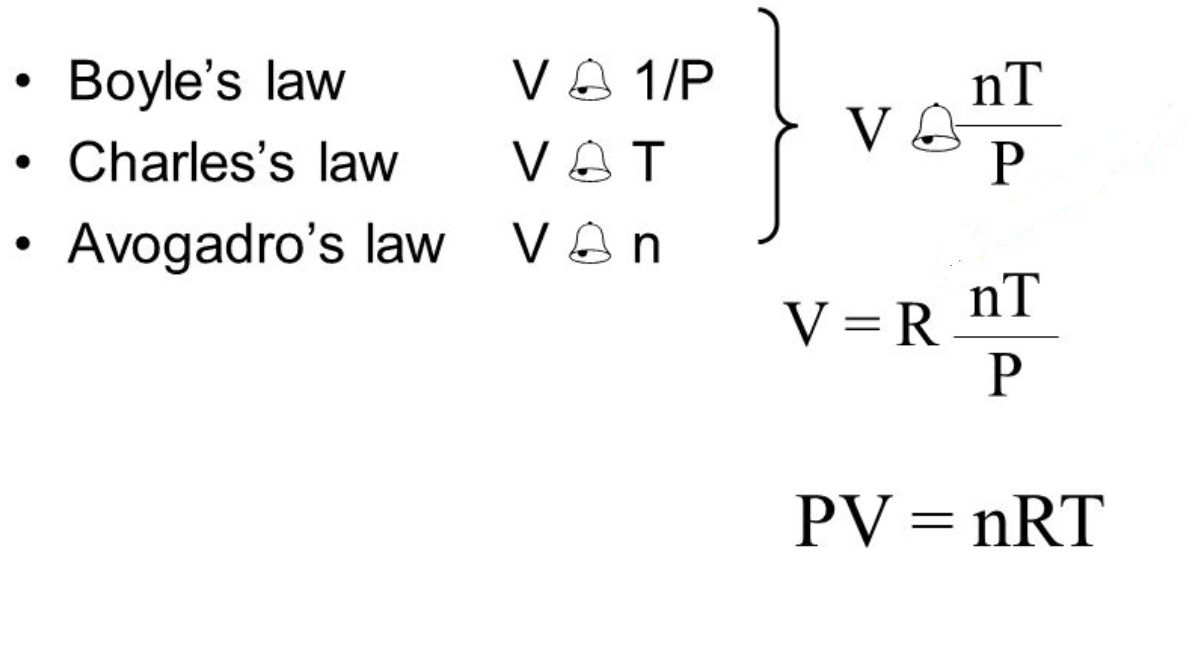
Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law. Boyle s law is one of the 4 gas laws each which describe the behavior of a sample of an ideal gas. Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law.
 Source: youtube.com
Source: youtube.com
The other three laws charles law gay lussac s law and avogadro s law can be combined with boyle s law to give you the ideal gas law an equation that describes the state of any hypothetical ideal gas. It is defined for an ideal gas. This experiment is carried out by inserting a certain amount of gas into a closed container. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume.
 Source: slideplayer.com
Source: slideplayer.com
This commonly involves explaining how the lung. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. The aim is simply to show how these laws relate to kinetic theory in a non mathematical way and to the ideal gas equation. At constant pressure you can say that the following relationship is true for an ideal gas. Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law.
 Source: pinterest.com
Source: pinterest.com
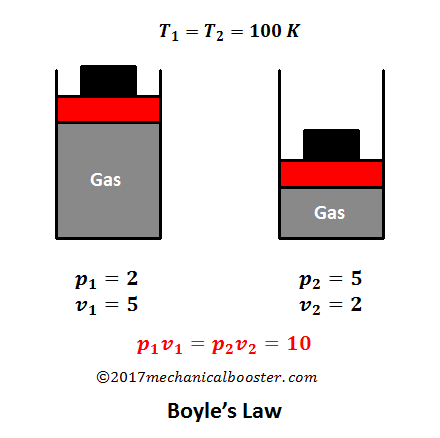
This page takes a simple look at boyle s law and charles law and is suitable for 16 18 year old chemistry students doing a course the equivalent of uk a level. V 1 6 kpa 10 l 3 kpa 20 l. This experiment is carried out by inserting a certain amount of gas into a closed container. According to boyle s law v 1 p 2 v 2 p 1. Ideal gas law makes use of a theoretical gas that has the following properties.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Boyle s law is one of the 4 gas laws each which describe the behavior of a sample of an ideal gas. Until a pretty good approach he found that if the gas. The other three laws charles law gay lussac s law and avogadro s law can be combined with boyle s law to give you the ideal gas law an equation that describes the state of any hypothetical ideal gas. Boyle s law charles s law gay lussac s law. This experiment is carried out by inserting a certain amount of gas into a closed container.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law. Boyle s law is a gas law. The aim is simply to show how these laws relate to kinetic theory in a non mathematical way and to the ideal gas equation. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. The other three laws charles law gay lussac s law and avogadro s law can be combined with boyle s law to give you the ideal gas law an equation that describes the state of any hypothetical ideal gas.
 Source: yumpu.com
Source: yumpu.com
Boyle s law charles s law and gay lussac s law form the combined gas law. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. Until a pretty good approach he found that if the gas. This commonly involves explaining how the lung. This equation called charles s law says that the ratio of volume to temperature v t will be conserved for an ideal gas all other factors.
 Source: mechanicalbooster.com
Source: mechanicalbooster.com
At constant pressure volume is proportional to absolute temperature. The other three laws charles law gay lussac s law and avogadro s law can be combined with boyle s law to give you the ideal gas law an equation that describes the state of any hypothetical ideal gas. This page takes a simple look at boyle s law and charles law and is suitable for 16 18 year old chemistry students doing a course the equivalent of uk a level. According to boyle s law v 1 p 2 v 2 p 1. This equation called charles s law says that the ratio of volume to temperature v t will be conserved for an ideal gas all other factors.
 Source: issuu.com
Source: issuu.com
Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume. The aim is simply to show how these laws relate to kinetic theory in a non mathematical way and to the ideal gas equation. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law. According to boyle s law v 1 p 2 v 2 p 1.
 Source: tes.com
Source: tes.com
Boyle s law is often used as part of an explanation on how the breathing system works in the human body. This equation called boyle s law says that all other factors being the same the product of pressure and volume pv will be conserved. V 1 6 kpa 10 l 3 kpa 20 l. Ideal gas law makes use of a theoretical gas that has the following properties. Show that boyle s law charles s law and avogadro s law can be derived from the ideal gas law.
 Source: studylib.net
Source: studylib.net
Until a pretty good approach he found that if the gas. This experiment is carried out by inserting a certain amount of gas into a closed container. Until a pretty good approach he found that if the gas. Robert boyle 1627 1691 conducted experiments to investigate the quantitative relationship between gas pressure and volume. The other three laws charles law gay lussac s law and avogadro s law can be combined with boyle s law to give you the ideal gas law an equation that describes the state of any hypothetical ideal gas.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title charles boyles law by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.