Common compounds of hydrogen
Common Compounds Of Hydrogen. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. Who when where. Any hydrocarbon will by definition contain hydrogen. Compounds of hydrogen.
 Examples Of Covalent Bonds And Compounds From thoughtco.com
Examples Of Covalent Bonds And Compounds From thoughtco.com
Water is one of the most abundant compounds of hydrogen and our earth s surface contains approximately 70 of ocean which is the major source of water. Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds. This category has the following 8 subcategories out of 8 total. Because hydrogen is located somewhat centrally in an electronegative sense it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. List of hydrogen carbonate compounds common compounds of hydrogen carbonate hco3 formula molecular weight. Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically.
Compounds of hydrogen.
Common substituents which in this way stabilize negative charge are esters aldehydes ketones acids. Wikimedia commons has media related to hydrogen compounds. However sea water contains many dissolved salts hence it can not be used directly. Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically. This category has the following 8 subcategories out of 8 total. Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds.
The element names in 10 different languages. Because hydrogen is located somewhat centrally in an electronegative sense it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. List of hydrogen compounds common compounds of hydrogen h formula molecular weight. Binary hydrogen compounds in group 1 are the ionic hydrides also called saline hydrides wherein hydrogen is bound electrostatically. Examples of a hydrocarbon include methane hexene butane and pentyne.

Who when where. Wikimedia commons has media related to hydrogen compounds. Up to 40 properties chemical physical. List of hydrogen carbonate compounds common compounds of hydrogen carbonate hco3 formula molecular weight. Any hydrocarbon will by definition contain hydrogen.
 Source: britannica.com
Source: britannica.com
Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically. Who when where. Active hydrogen compounds are those in which the substituents present are capable of stabilizing the conjugate base formed on deprotonation of the starting material. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. List of hydrogen compounds common compounds of hydrogen h formula molecular weight.
 Source: boomeria.org
Source: boomeria.org
Over 3 600 nuclides isotopes. Any hydrocarbon will by definition contain hydrogen. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. Compounds of hydrogen. Examples of a hydrocarbon include methane hexene butane and pentyne.
 Source: pinterest.ph
Source: pinterest.ph
Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds. Compounds of hydrogen. However sea water contains many dissolved salts hence it can not be used directly. Active hydrogen compounds are those in which the substituents present are capable of stabilizing the conjugate base formed on deprotonation of the starting material. Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically.
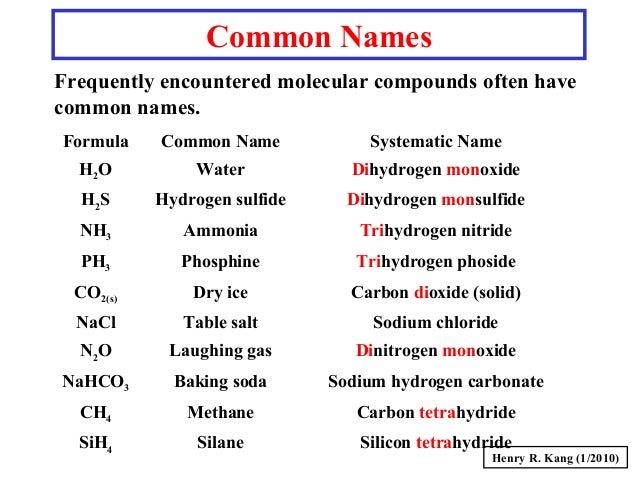 Source: slideshare.net
Source: slideshare.net
There are three ty. There are three ty. However sea water contains many dissolved salts hence it can not be used directly. List of hydrogen compounds common compounds of hydrogen h formula molecular weight. Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds.
Source: quora.com
Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically. Hydrogen compounds are those chemical compounds which contain hydrogen. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. The element names in 10 different languages. Over 4 400 nuclide decay modes.
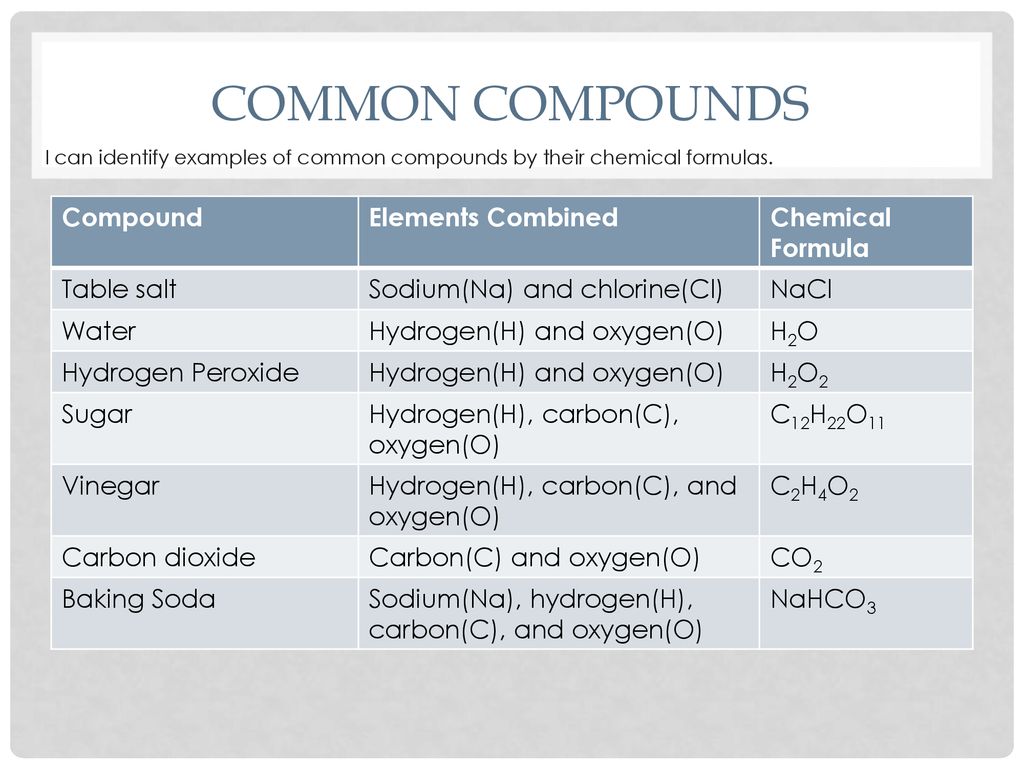 Source: slideplayer.com
Source: slideplayer.com
In addition chemistry and technical terms are linked to their definitions in the site s chemistry and environmental dictionary. List of hydrogen compounds common compounds of hydrogen h formula molecular weight. The element names in 10 different languages. Water is one of the most abundant compounds of hydrogen and our earth s surface contains approximately 70 of ocean which is the major source of water. Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds.
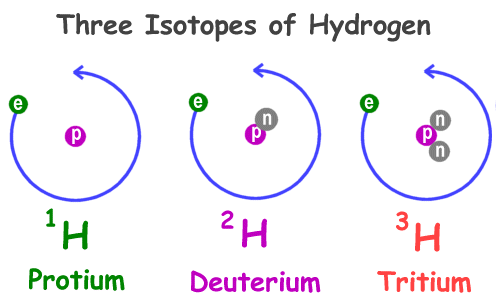 Source: ducksters.com
Source: ducksters.com
Because hydrogen is located somewhat centrally in an electronegative sense it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. Who when where. Over 3 600 nuclides isotopes. Binary hydrogen compounds in group 1 are the ionic hydrides also called saline hydrides wherein hydrogen is bound electrostatically. Examples of a hydrocarbon include methane hexene butane and pentyne.
 Source: thoughtco.com
Source: thoughtco.com
Over 3 600 nuclides isotopes. However sea water contains many dissolved salts hence it can not be used directly. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. Water is one of the most abundant compounds of hydrogen and our earth s surface contains approximately 70 of ocean which is the major source of water. Over 4 400 nuclide decay modes.
 Source: livescience.com
Source: livescience.com
The element names in 10 different languages. List of hydrogen compounds common compounds of hydrogen h formula molecular weight. List of hydrogen carbonate compounds common compounds of hydrogen carbonate hco3 formula molecular weight. Common substituents which in this way stabilize negative charge are esters aldehydes ketones acids. Binary hydrogen compounds in group 1 are the ionic hydrides also called saline hydrides wherein hydrogen is bound electrostatically.
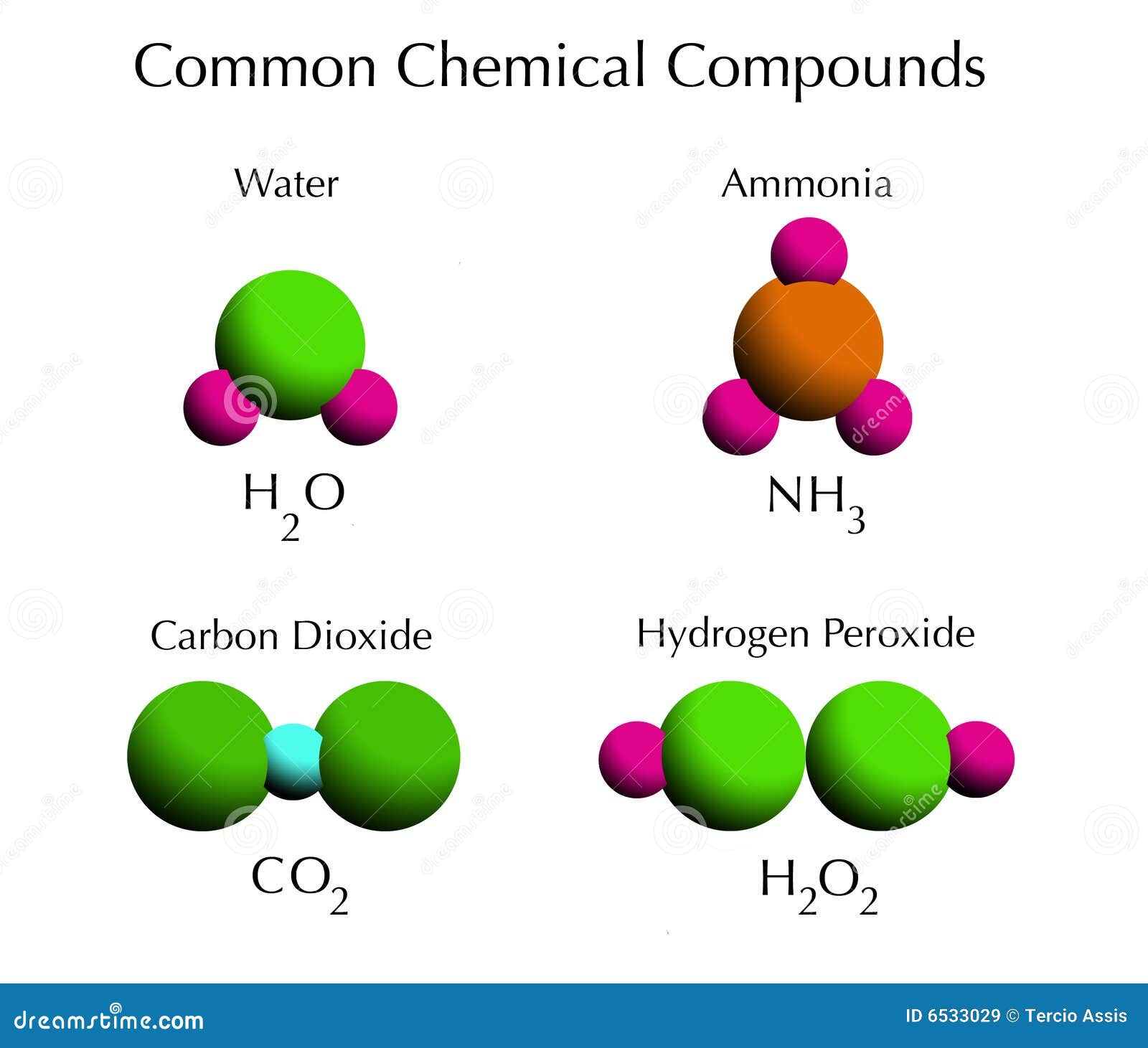 Source: dreamstime.com
Source: dreamstime.com
The element names in 10 different languages. Water is one of the most abundant compounds of hydrogen and our earth s surface contains approximately 70 of ocean which is the major source of water. Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically. Wikimedia commons has media related to hydrogen compounds. Over 4 400 nuclide decay modes.
 Source: sciencedirect.com
Source: sciencedirect.com
Water is essential for all living things and our body contains about 65 water. In addition chemistry and technical terms are linked to their definitions in the site s chemistry and environmental dictionary. Stabilization of the resultant anion significantly enhances the acidity of the proton hence active hydrogen compounds. Examples of a hydrocarbon include methane hexene butane and pentyne. There are three ty.
 Source: slideplayer.com
Source: slideplayer.com
Wikimedia commons has media related to hydrogen compounds. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. Active hydrogen compounds are those in which the substituents present are capable of stabilizing the conjugate base formed on deprotonation of the starting material. This category has the following 8 subcategories out of 8 total. Because hydrogen is located somewhat centrally in an electronegative sense it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic.
 Source: pinterest.co.uk
Source: pinterest.co.uk
Over 4 400 nuclide decay modes. All hydrocarbons are basically a chain of carbons in some fashion with hydrogens bonded to the carbons. Any hydrocarbon will by definition contain hydrogen. Hydrides the term hydride is used to indicate compounds of the type m x h y and not necessarily to indicate that any compounds listed behave as hydrides chemically. Examples of a hydrocarbon include methane hexene butane and pentyne.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title common compounds of hydrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.