Densities of liquids
Densities Of Liquids. This means that the value of density is independent of the quantity of matter present. Density of some common liquids. Ethanol grain alcohol 0 810. Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity.
 Reduced Temperature Reduced Density Plot Of Saturated Liquid Densities Download Scientific Diagram From researchgate.net
Reduced Temperature Reduced Density Plot Of Saturated Liquid Densities Download Scientific Diagram From researchgate.net
Liquids even two liquids that look similar can be distinguished by their densities. In general densities of liquids are known more precisely than those of solids. Also densities of elements and compounds are known more precisely than densities of materials with variable compositions such as wood or robber. Because these liquids will have different densities there will be different layers that are visible sort of like being able to see ice cubes frozen water in room temperature water. Nitrogen at stp 0 001251. Density d is calculated by dividing an object s mass m by its volume v.
In liquids the density determines whether or not something will sink or float in that liquid.
Because these liquids will have different densities there will be different layers that are visible sort of like being able to see ice cubes frozen water in room temperature water. Carbon monoxide at stp 0 00125. Slightly less than the solid. If a liquid that is less dense than water is gently added to the surface of the water it will float on the water. Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity. Density density in g cm 3.
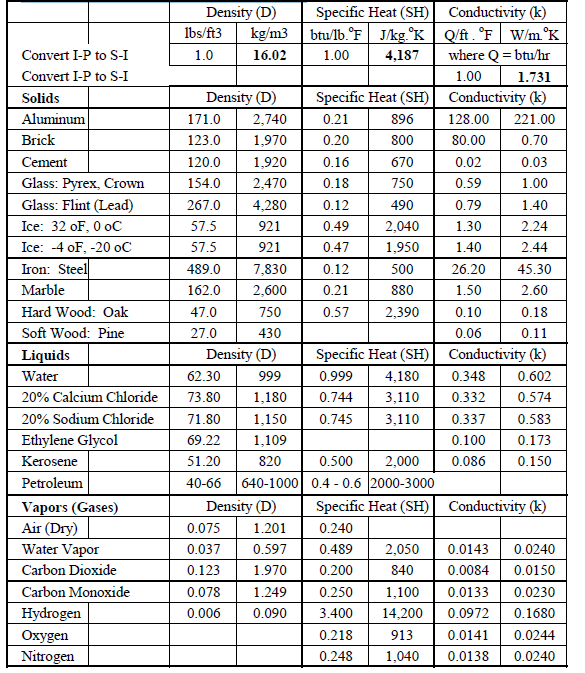 Source: energy-models.com
Source: energy-models.com
Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity. Carbon dioxide at stp 0 001977. Density frac mass volume the units of density are commonly expressed as g cm 3 for solids g ml for liquids and g l for gases. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense. In general densities of liquids are known more precisely than those of solids.
 Source: inspirationlaboratories.com
Source: inspirationlaboratories.com
The density of a liquid can be found by determining the mass of a sample of the liquid and dividing it by the volume. A substance whether it s a liquid or otherwise with a greater density than. Liquids even two liquids that look similar can be distinguished by their densities. Because these liquids will have different densities there will be different layers that are visible sort of like being able to see ice cubes frozen water in room temperature water. Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity.
 Source: sciencenotes.org
Source: sciencenotes.org
In liquids the density determines whether or not something will sink or float in that liquid. Carbon monoxide at stp 0 00125. In liquids the density determines whether or not something will sink or float in that liquid. Density g cm 3 state of matter. Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity.
 Source: sciencephoto.com
Source: sciencephoto.com
Density frac mass volume the units of density are commonly expressed as g cm 3 for solids g ml for liquids and g l for gases. Density d is calculated by dividing an object s mass m by its volume v. Density density in g cm 3. This means that the value of density is independent of the quantity of matter present. Carbon monoxide at stp 0 00125.
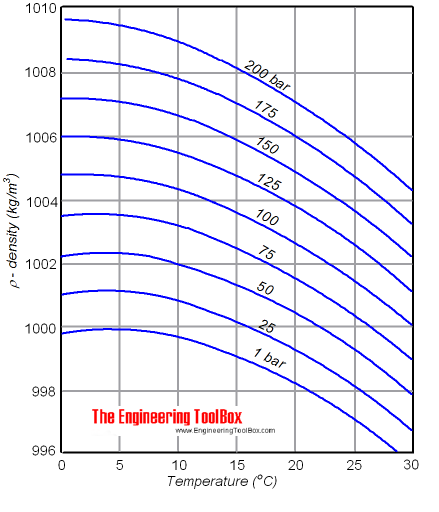 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Slightly further apart than a solid. Nitrogen at stp 0 001251. In liquids the density determines whether or not something will sink or float in that liquid. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense. Density is determined by dividing the mass of a substance by its volume.
 Source: researchgate.net
Source: researchgate.net
Density is also an intensive property of matter. Nitrogen at stp 0 001251. Density density in g cm 3. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense. 1 kg m3 0 001 g cm3 0 0005780 oz in3 0 16036 oz gal imperial 0 1335 oz gal u s 0 0624 lb ft3 0 000036127 lb in3 1 6856 lb yd3 0 010022 lb gal imperial 0 008345 lb gal u s 0 0007525 ton yd3.
 Source: slideshare.net
Source: slideshare.net
The density of a liquid is a measure of how heavy it is for the amount measured. Helium at stp 0 000178. Density is also an intensive property of matter. Mostly in the range of a few grams per liter. The density of a liquid can be found by determining the mass of a sample of the liquid and dividing it by the volume.
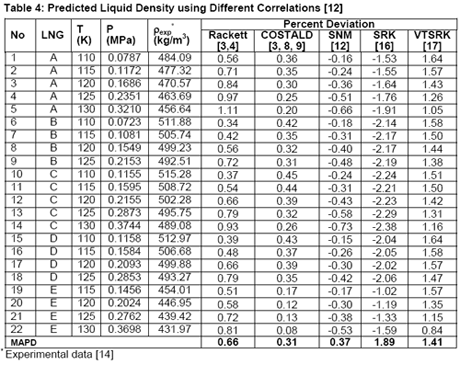 Source: jmcampbell.com
Source: jmcampbell.com
Density frac mass volume the units of density are commonly expressed as g cm 3 for solids g ml for liquids and g l for gases. If a liquid that is less dense than water is gently added to the surface of the water it will float on the water. Air at stp 0 001293. In liquids the density determines whether or not something will sink or float in that liquid. Slightly less than the solid.
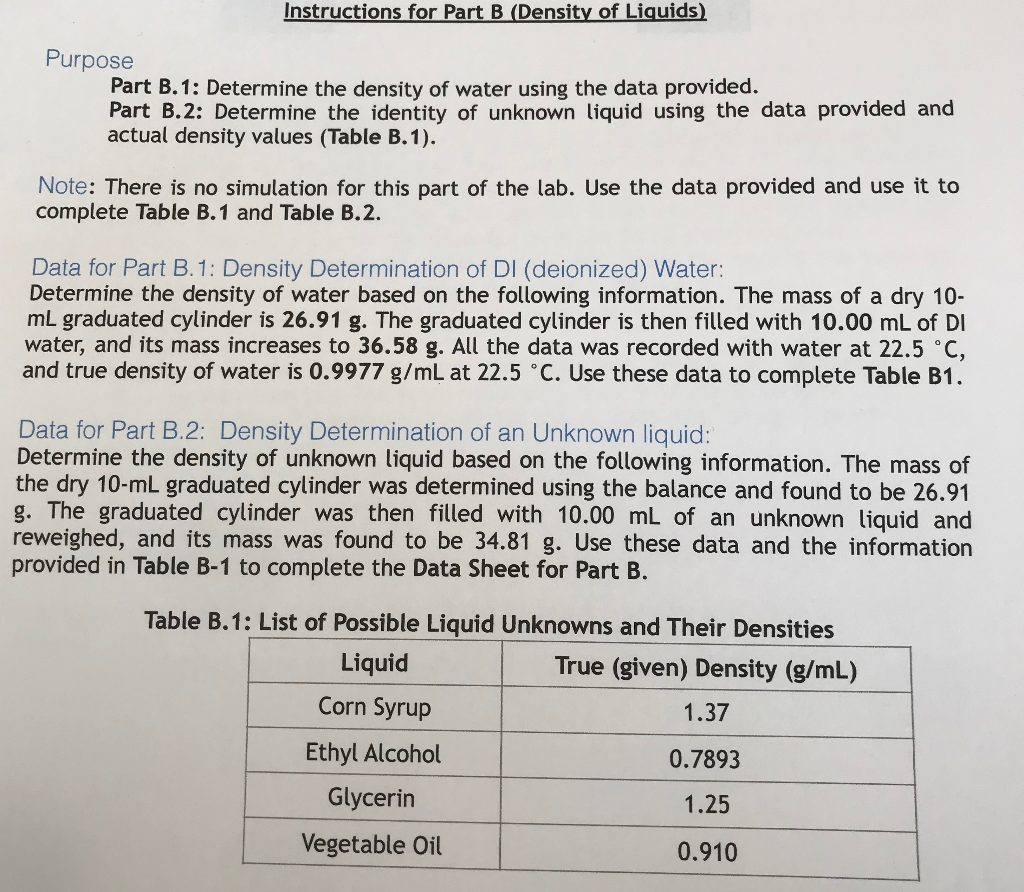 Source: chegg.com
Source: chegg.com
Helium at stp 0 000178. Density of some common liquids. If a liquid that is less dense than water is gently added to the surface of the water it will float on the water. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense. Liquids even two liquids that look similar can be distinguished by their densities.
 Source: jmcampbell.com
Source: jmcampbell.com
Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity. Liquids even two liquids that look similar can be distinguished by their densities. Because these liquids will have different densities there will be different layers that are visible sort of like being able to see ice cubes frozen water in room temperature water. A substance whether it s a liquid or otherwise with a greater density than. 1 kg m3 0 001 g cm3 0 0005780 oz in3 0 16036 oz gal imperial 0 1335 oz gal u s 0 0624 lb ft3 0 000036127 lb in3 1 6856 lb yd3 0 010022 lb gal imperial 0 008345 lb gal u s 0 0007525 ton yd3.
 Source: reddit.com
Source: reddit.com
Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity. Slightly further apart than a solid. Take for example water. Density is also an intensive property of matter. If a liquid that is less dense than water is gently added to the surface of the water it will float on the water.
 Source: jmcampbell.com
Source: jmcampbell.com
Nitrogen at stp 0 001251. A substance whether it s a liquid or otherwise with a greater density than. Density d is calculated by dividing an object s mass m by its volume v. Because these liquids will have different densities there will be different layers that are visible sort of like being able to see ice cubes frozen water in room temperature water. 1 kg m3 0 001 g cm3 0 0005780 oz in3 0 16036 oz gal imperial 0 1335 oz gal u s 0 0624 lb ft3 0 000036127 lb in3 1 6856 lb yd3 0 010022 lb gal imperial 0 008345 lb gal u s 0 0007525 ton yd3.
Source: gssd.ca
Liquids even two liquids that look similar can be distinguished by their densities. Helium at stp 0 000178. Where ρ l is the liquid density ρ g is the gas density φ l mass sources representing the inflow of liquid from a reservoir to the wellbore φ g mass sources representing the inflow of gas from a reservoir to the pipe h is the liquid volume fraction s is the coordinate along the length of the well t is time u gas and liquid are traveling with the same velocity. Pour 150 ml of water into beaker 1 150 ml of corn syrup into beaker 2 and 150 ml of vegetable oil into beaker 3. Solid iron 7 8.
 Source: pinterest.com
Source: pinterest.com
Nitrogen at stp 0 001251. Pour 150 ml of water into beaker 1 150 ml of corn syrup into beaker 2 and 150 ml of vegetable oil into beaker 3. In liquids the density determines whether or not something will sink or float in that liquid. Density frac mass volume the units of density are commonly expressed as g cm 3 for solids g ml for liquids and g l for gases. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense.
 Source: instructables.com
Source: instructables.com
The density of a liquid can be found by determining the mass of a sample of the liquid and dividing it by the volume. If you weigh equal amounts or volumes of two different liquids the liquid that weighs more is more dense. Air at stp 0 001293. Density of some common liquids. Nitrogen at stp 0 001251.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title densities of liquids by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







