Describe alkali metals
Describe Alkali Metals. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process. All elements in the alkali metal group occur in nature. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. They include lithium sodium potassium rubidium cesium and francium.
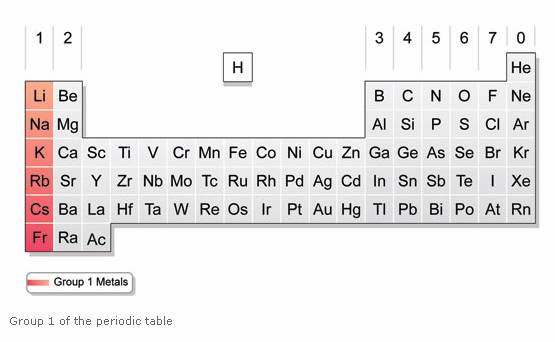 Alkali Metals Solutions Examples Reactions Videos From onlinemathlearning.com
Alkali Metals Solutions Examples Reactions Videos From onlinemathlearning.com
In general alkali refers to the basic or alkaline nature of their metal hydroxides. Alkali metals are any of the elements found in group ia of the periodic table the first column. The alkali metals are so called because reaction with water forms alkalies i e strong bases capable of neutralizing acids. Any of a group of soft metallic elements that form alkali solutions when they combine with water. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process.
Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points.
Although hydrogen is in group 1 and also in group 17 it is a nonmetal and deserves separate consideration later in this tutorial. They include lithium sodium potassium rubidium cesium and francium. The alkali metals are lithium sodium potassium rubidium cesium and francium. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points.
 Source: docbrown.info
Source: docbrown.info
The alkali metals the alkali metals lithium sodium potassium rubidium cesium and francium constitute group 1 of the periodic table. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. Alkali metal alkali metal chemical properties. Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. The hydroxides possess strong basic properties.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process. They react with water to produce an alkaline metal hydroxide solution and hydrogen. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. Alkali metals are any of the elements found in group ia of the periodic table the first column.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Lithium is the only alkali metal that reacts slowly with water. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. Alkali metals have a corresponding noble gas ns 1 electronic configuration. The reaction is so vigorous in nature that the hydrogen gas produced during the reaction catches fire. Alkali metal properties the alkali metals exhibit many of the physical properties common to metals although their densities are lower than those of other metals.
 Source: emedicalprep.com
Source: emedicalprep.com
The hydroxides possess strong basic properties. Alkali metal alkali metal chemical properties. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. Any of a group of soft metallic elements that form alkali solutions when they combine with water. The alkali metals are so called because reaction with water forms alkalies i e strong bases capable of neutralizing acids.
 Source: chem4kids.com
Source: chem4kids.com
All elements in the alkali metal group occur in nature. The alkali metals are so called because reaction with water forms alkalies i e strong bases capable of neutralizing acids. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. The hydroxides possess strong basic properties.
 Source: slideplayer.com
Source: slideplayer.com
Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Lithium is the only alkali metal that reacts slowly with water. Alkali metal any of the six chemical elements that make up group 1 ia of the periodic table namely lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process. Except for cesium which has a gold sheen alkali metals are white.
 Source: britannica.com
Source: britannica.com
Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. Alkali metal any of the six chemical elements that make up group 1 ia of the periodic table namely lithium li sodium na potassium k rubidium rb cesium cs and francium fr. All elements in the alkali metal group occur in nature. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table.

In general alkali refers to the basic or alkaline nature of their metal hydroxides. Alkali metal alkali metal chemical properties. Lithium is the only alkali metal that reacts slowly with water. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. All elements in the alkali metal group occur in nature.
 Source: thoughtco.com
Source: thoughtco.com
They react with water to produce an alkaline metal hydroxide solution and hydrogen. The hydroxides possess strong basic properties. Alkali metals are any of the elements found in group ia of the periodic table the first column. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process. They react with water to produce an alkaline metal hydroxide solution and hydrogen.
 Source: chegg.com
Source: chegg.com
Alkali metal alkali metal chemical properties. Any of a group of soft metallic elements that form alkali solutions when they combine with water. All elements in the alkali metal group occur in nature. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids. Alkali metals are any of the elements found in group ia of the periodic table the first column.
 Source: study.com
Source: study.com
The alkali metals are lithium sodium potassium rubidium cesium and francium. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids. Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table.
 Source: richmond.k12.nc.us
Source: richmond.k12.nc.us
Alkali metal any of the six chemical elements that make up group 1 ia of the periodic table namely lithium li sodium na potassium k rubidium rb cesium cs and francium fr. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. Except for cesium which has a gold sheen alkali metals are white. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table.
 Source: studylib.net
Source: studylib.net
The hydroxides possess strong basic properties. The hydroxides possess strong basic properties. Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. Except for cesium which has a gold sheen alkali metals are white.
 Source: issuu.com
Source: issuu.com
Alkali metal properties the alkali metals exhibit many of the physical properties common to metals although their densities are lower than those of other metals. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. Group 1 the alkali metals the group 1 elements are all soft reactive metals with low melting points. Any of a group of soft metallic elements that form alkali solutions when they combine with water. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group.
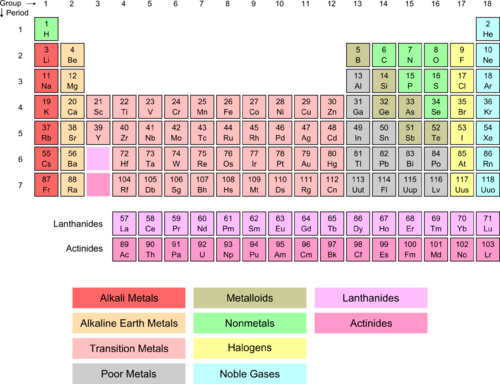 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. Any of a group of soft metallic elements that form alkali solutions when they combine with water. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Except for cesium which has a gold sheen alkali metals are white. Lithium is the only alkali metal that reacts slowly with water.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title describe alkali metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.