Different indicators of acids and bases
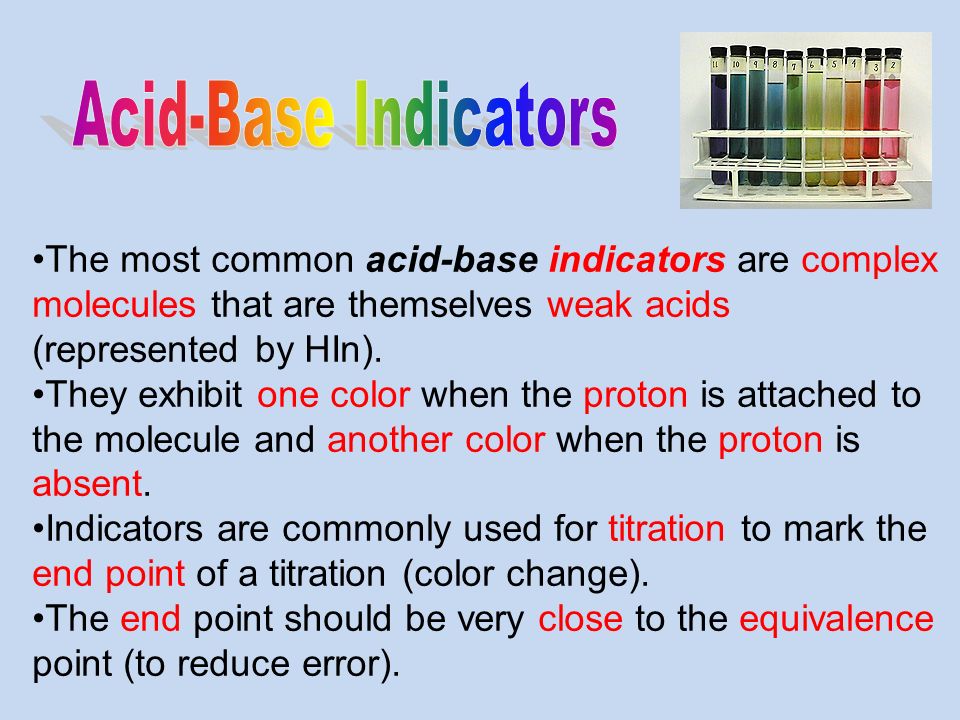
Different Indicators Of Acids And Bases. Indicators as weak acids. There will be an equilibrium established when this acid dissolves in water. An indicator tells us whether the substance we are testing is acidic or basic by change in its colour. The quantity of indicator in aqueous aq or alcohol alc solution is specified.
 Acid Base Indicators Year 8 Science From sites.google.com
Acid Base Indicators Year 8 Science From sites.google.com
An indicator gives different colours in acid and base. It has a seriously complicated molecule which we will simplify to hlit. An indicator is a dye that changes colour when it is put into an acid or a base. Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges. Phenolphthalein is an indicator of acids colorless and bases pink. The lit is the rest of the weak acid molecule.
Litmus is a weak acid.
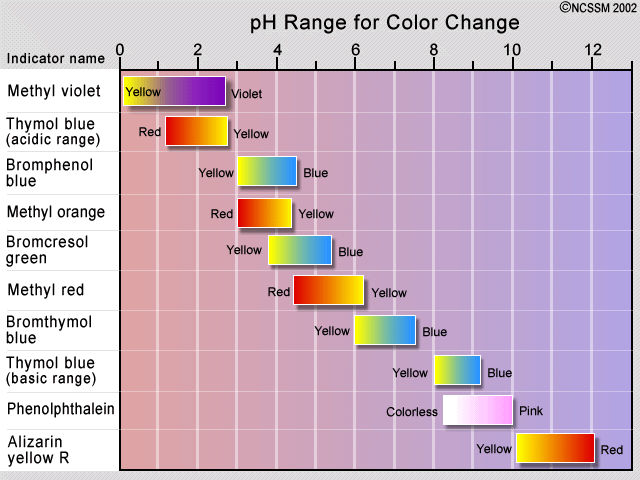
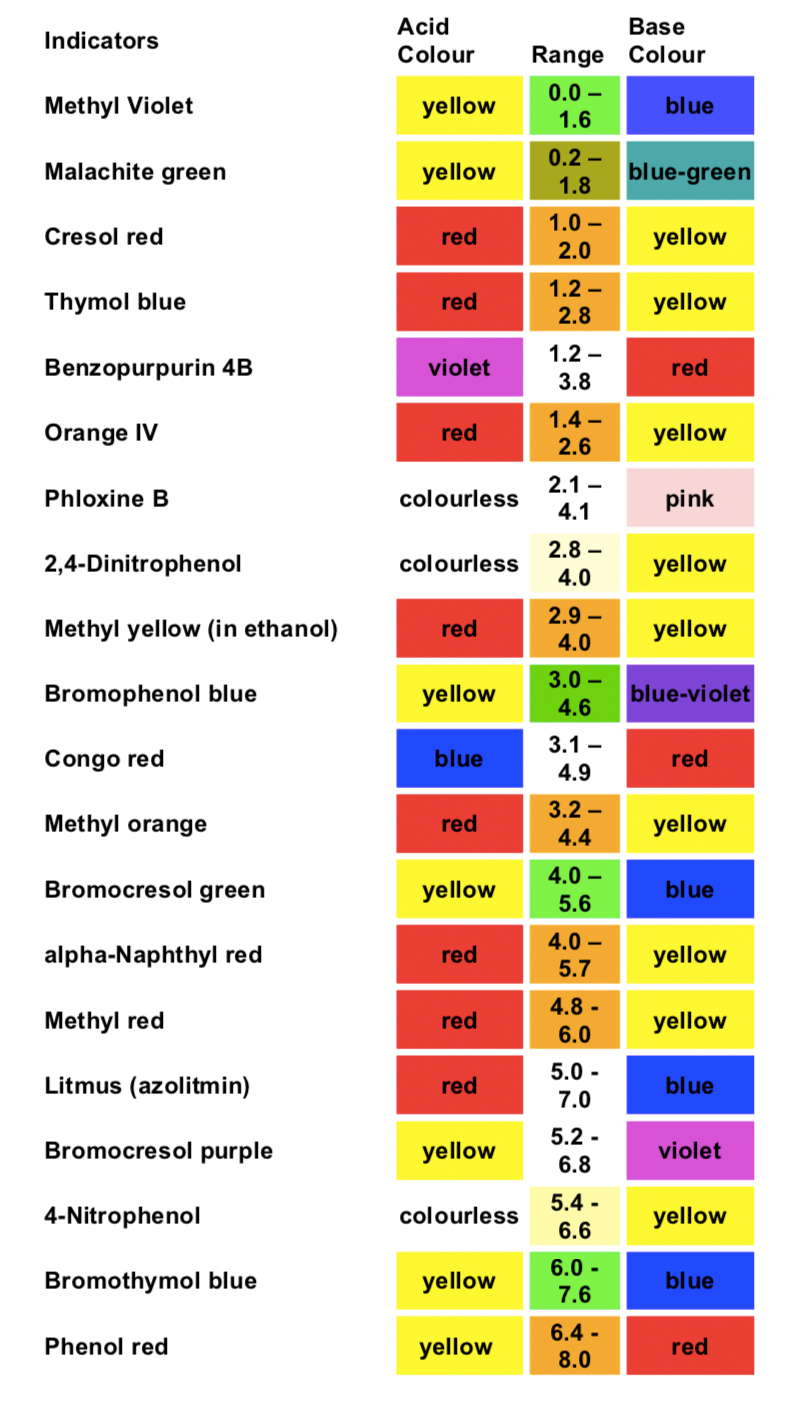
An indicator gives different colours in acid and base. An indicator tells us whether the substance we are testing is acidic or basic by change in its colour. Indicators as weak acids. Tried and true indicators include thymol blue tropeolin oo methyl yellow methyl orange bromphenol blue bromcresol green methyl red bromthymol blue phenol red neutral red phenolphthalein thymolphthalein alizarin yellow tropeolin o nitramine and. Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic. Litmus is a weak acid.
 Source: foundoutaboutchemistry.blogspot.com
Source: foundoutaboutchemistry.blogspot.com
Common acid base indicators. Phenolphthalein is an indicator of acids colorless and bases pink. Litmus is a weak acid. Indicators as weak acids. A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges. Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges. Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic. Common acid base indicators. Tried and true indicators include thymol blue tropeolin oo methyl yellow methyl orange bromphenol blue bromcresol green methyl red bromthymol blue phenol red neutral red phenolphthalein thymolphthalein alizarin yellow tropeolin o nitramine and.
 Source: study.com
Source: study.com
Phenolphthalein is an indicator of acids colorless and bases pink. Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic. Tried and true indicators include thymol blue tropeolin oo methyl yellow methyl orange bromphenol blue bromcresol green methyl red bromthymol blue phenol red neutral red phenolphthalein thymolphthalein alizarin yellow tropeolin o nitramine and. The h is the proton which can be given away to something else. Phenolphthalein is an indicator of acids colorless and bases pink.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Common acid base indicators. The quantity of indicator in aqueous aq or alcohol alc solution is specified. Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to hlit. An indicator is a dye that changes colour when it is put into an acid or a base.
 Source: slideplayer.com
Source: slideplayer.com
The h is the proton which can be given away to something else. An indicator is a dye that changes colour when it is put into an acid or a base. Litmus is a weak acid. It has a seriously complicated molecule which we will simplify to hlit. There will be an equilibrium established when this acid dissolves in water.
 Source: nigerianscholars.com
Source: nigerianscholars.com
An indicator tells us whether the substance we are testing is acidic or basic by change in its colour. Common acid base indicators. A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic. An indicator gives different colours in acid and base. Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges.
 Source: sites.google.com
Source: sites.google.com
Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic. It has a seriously complicated molecule which we will simplify to hlit. The h is the proton which can be given away to something else. A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic. Phenolphthalein is an indicator of acids colorless and bases pink.
 Source: slideplayer.com
Source: slideplayer.com
It has a seriously complicated molecule which we will simplify to hlit. A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic. Phenolphthalein is an indicator of acids colorless and bases pink. An indicator is a dye that changes colour when it is put into an acid or a base. Indicators as weak acids.
 Source: m.youtube.com
Source: m.youtube.com
An indicator gives different colours in acid and base. Phenolphthalein is an indicator of acids colorless and bases pink. The h is the proton which can be given away to something else. There will be an equilibrium established when this acid dissolves in water. An indicator tells us whether the substance we are testing is acidic or basic by change in its colour.
 Source: compoundchem.com
Source: compoundchem.com
It has a seriously complicated molecule which we will simplify to hlit. An indicator tells us whether the substance we are testing is acidic or basic by change in its colour. Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic. The h is the proton which can be given away to something else. There will be an equilibrium established when this acid dissolves in water.
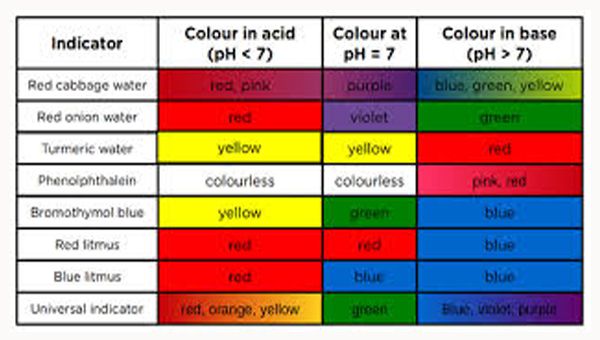 Source: guidancecorner.com
Source: guidancecorner.com
Phenolphthalein is an indicator of acids colorless and bases pink. An indicator tells us whether the substance we are testing is acidic or basic by change in its colour. There will be an equilibrium established when this acid dissolves in water. The h is the proton which can be given away to something else. It has a seriously complicated molecule which we will simplify to hlit.
 Source: unacademy.com
Source: unacademy.com
Phenolphthalein is an indicator of acids colorless and bases pink. There will be an equilibrium established when this acid dissolves in water. The quantity of indicator in aqueous aq or alcohol alc solution is specified. Indicators as weak acids. The h is the proton which can be given away to something else.
 Source: quora.com
Source: quora.com
Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges. There will be an equilibrium established when this acid dissolves in water. Tried and true indicators include thymol blue tropeolin oo methyl yellow methyl orange bromphenol blue bromcresol green methyl red bromthymol blue phenol red neutral red phenolphthalein thymolphthalein alizarin yellow tropeolin o nitramine and. Several acid base indicators are listed below some more than once if they can be used over multiple ph ranges. The lit is the rest of the weak acid molecule.
 Source: brainly.in
Source: brainly.in
The quantity of indicator in aqueous aq or alcohol alc solution is specified. The quantity of indicator in aqueous aq or alcohol alc solution is specified. An indicator is a dye that changes colour when it is put into an acid or a base. Common acid base indicators. Sodium hydroxide is a base and it was in the pitcher at the beginning so when added to the phenolphthalein in beakers 2 and 4 it turned pink top half of the graphic.
 Source: thoughtco.com
Source: thoughtco.com
Indicators as weak acids. There will be an equilibrium established when this acid dissolves in water. Phenolphthalein is an indicator of acids colorless and bases pink. The lit is the rest of the weak acid molecule. The h is the proton which can be given away to something else.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title different indicators of acids and bases by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.