Do alkaline earth metals react with water
Do Alkaline Earth Metals React With Water. Magnesium and beryllium do not react with water. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. The reactivity of the. The speed and violence.
 Reactions Of Alkaline Earth Metals With Water Youtube From youtube.com
Reactions Of Alkaline Earth Metals With Water Youtube From youtube.com
You know alkaline earth metals group 2 elements are not reactive like alkali metals. In each reaction hydrogen gas is given off and the metal hydroxide is produced. The speed and violence. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. Therfore their reactivity with water is less compared to alkali metals. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two.
They tend to lose these electrons easily as they re sort of a loose end the metal is energeti.
Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. You know alkaline earth metals group 2 elements are not reactive like alkali metals. In each reaction hydrogen gas is given off and the metal hydroxide is produced. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. The speed and violence. From alkaline earth metals calcium strontium and barium reacts with water.
 Source: slideplayer.com
Source: slideplayer.com
Therfore their reactivity with water is less compared to alkali metals. You know alkaline earth metals group 2 elements are not reactive like alkali metals. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. From alkaline earth metals calcium strontium and barium reacts with water. Therfore their reactivity with water is less compared to alkali metals.
 Source: hebasoffar.blogspot.com
Source: hebasoffar.blogspot.com
You know alkaline earth metals group 2 elements are not reactive like alkali metals. From alkaline earth metals calcium strontium and barium reacts with water. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. You know alkaline earth metals group 2 elements are not reactive like alkali metals. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h.
 Source: slideplayer.com
Source: slideplayer.com
Reactions of alkali metals with water all the alkali metals react vigorously with cold water. In each reaction hydrogen gas is given off and the metal hydroxide is produced. From alkaline earth metals calcium strontium and barium reacts with water. You know alkaline earth metals group 2 elements are not reactive like alkali metals. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Therfore their reactivity with water is less compared to alkali metals. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. Therfore their reactivity with water is less compared to alkali metals. From alkaline earth metals calcium strontium and barium reacts with water. Magnesium and beryllium do not react with water.
 Source: studylib.net
Source: studylib.net
Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. The speed and violence. Magnesium and beryllium do not react with water. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. You know alkaline earth metals group 2 elements are not reactive like alkali metals.
 Source: slideplayer.com
Source: slideplayer.com
Reactions of alkali metals with water all the alkali metals react vigorously with cold water. They tend to lose these electrons easily as they re sort of a loose end the metal is energeti. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. Magnesium and beryllium do not react with water. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h.
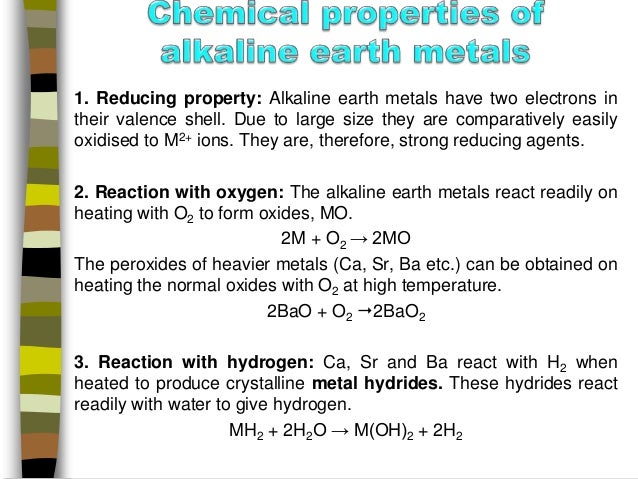 Source: slideshare.net
Source: slideshare.net
They tend to lose these electrons easily as they re sort of a loose end the metal is energeti. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. The reactivity of the. In each reaction hydrogen gas is given off and the metal hydroxide is produced. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h.
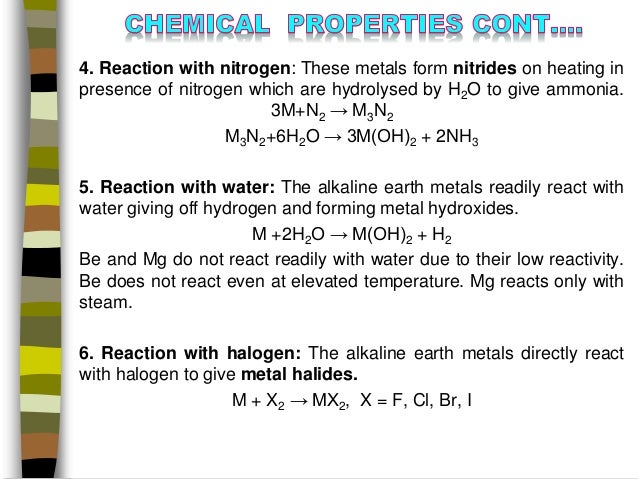 Source: slideshare.net
Source: slideshare.net
The reactivity of the. From alkaline earth metals calcium strontium and barium reacts with water. You know alkaline earth metals group 2 elements are not reactive like alkali metals. The speed and violence. The reactivity of the.
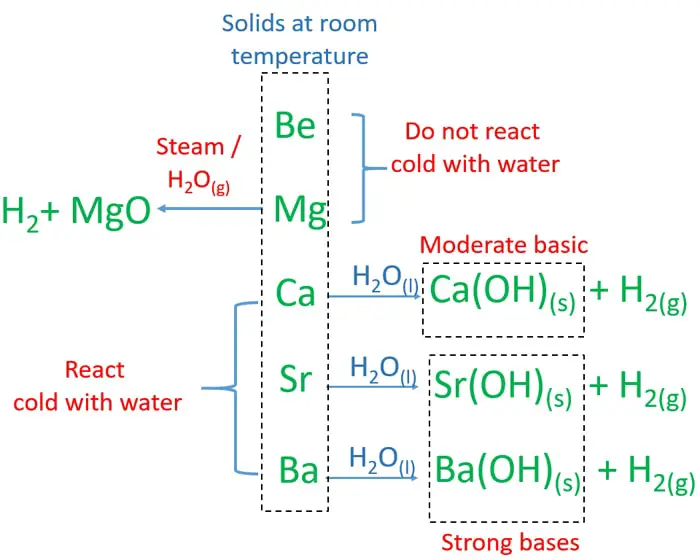 Source: chemistryscl.com
Source: chemistryscl.com
Magnesium and beryllium do not react with water. You know alkaline earth metals group 2 elements are not reactive like alkali metals. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. They tend to lose these electrons easily as they re sort of a loose end the metal is energeti. The speed and violence.
 Source: youtube.com
Source: youtube.com
You know alkaline earth metals group 2 elements are not reactive like alkali metals. In each reaction hydrogen gas is given off and the metal hydroxide is produced. Magnesium and beryllium do not react with water. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. They tend to lose these electrons easily as they re sort of a loose end the metal is energeti.
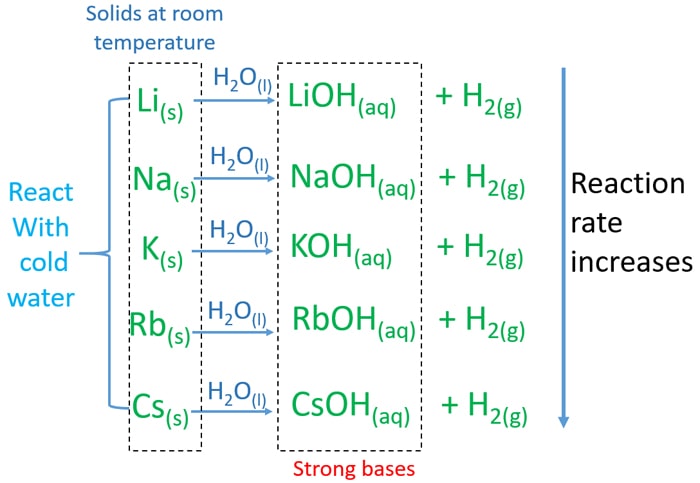 Source: chemistryscl.com
Source: chemistryscl.com
From alkaline earth metals calcium strontium and barium reacts with water. They tend to lose these electrons easily as they re sort of a loose end the metal is energeti. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. In each reaction hydrogen gas is given off and the metal hydroxide is produced. From alkaline earth metals calcium strontium and barium reacts with water.
 Source: ehs.stanford.edu
Source: ehs.stanford.edu
You know alkaline earth metals group 2 elements are not reactive like alkali metals. The speed and violence. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. Reactions of alkali metals with water all the alkali metals react vigorously with cold water.
 Source: youtube.com
Source: youtube.com
From alkaline earth metals calcium strontium and barium reacts with water. The speed and violence. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. The reactivity of the. Magnesium and beryllium do not react with water.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Most of the alkaline earth metals react with water to produce metal hydroxides which are compounds containing the hydroxide ion which is oh o h. Therfore their reactivity with water is less compared to alkali metals. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. Alkali earth metals are in the second column of the periodic table so they have two valence electrons alkali metals have one earth alkali has two. The speed and violence.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The reactivity of the. The reactivity of the. The speed and violence. Reactions of alkali metals with water all the alkali metals react vigorously with cold water. From alkaline earth metals calcium strontium and barium reacts with water.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title do alkaline earth metals react with water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





