Does copper react with aluminum
Does Copper React With Aluminum. Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener. Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. How liquid metal affects copper nickel and aluminum corrosion test. Aluminium reacts with oxygen forming a protective layer of alumnium iii oxide that prevents further reaction with oxygen.
 Copper Ii Sulphate And Aluminium Reaction Youtube From m.youtube.com
Copper Ii Sulphate And Aluminium Reaction Youtube From m.youtube.com
This type of reaction is known as an oxidation reduction reaction or a redox reaction. Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. During the reaction between copper ii chloride and aluminum the aluminum dissolves to create a solution with aluminum ions with a 3 charge and copper metal. In the aluminum half reaction the aluminum ion has a charge of positive three. The product in this reaction is also alumnium iii oxide.
Like magnesium aluminium burns in oxygen with a brilliant white flame.
Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy. Galvanic reaction chart below is a galvanic reaction chart for dissimilar metals. The individual reductions or oxidations are called half reactions. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. The aluminum gains this charge by losing electrons during the reaction.
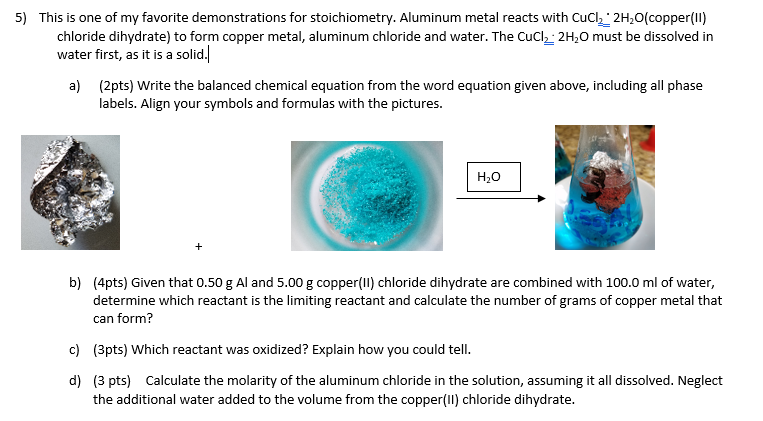 Source: chegg.com
Source: chegg.com
Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. Hot water discharge pipes should be extended beyond the roof preferably to ground. Aluminum metal is always covered in a thin but protective layer of aluminum oxide al 2. 4 al s 3 o 2 g 2 al 2 o 3 s. Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener.
 Source: youtube.com
Source: youtube.com
Like magnesium aluminium burns in oxygen with a brilliant white flame. But that isn t the whole story. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. Copper ii ions will hydrolyzeto produce an excess of hydrogen ions making the copper ii chloride solution slightly acidic. The aluminum gains this charge by losing electrons during the reaction.
 Source: m.youtube.com
Source: m.youtube.com
The individual reductions or oxidations are called half reactions. Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener. In the aluminum half reaction the aluminum ion has a charge of positive three. Like magnesium aluminium burns in oxygen with a brilliant white flame. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows.
 Source: m.youtube.com
Source: m.youtube.com
How liquid metal affects copper nickel and aluminum corrosion test. 4 al s 3 o 2 g 2 al 2 o 3 s. How liquid metal affects copper nickel and aluminum corrosion test. Aluminium reacts with oxygen forming a protective layer of alumnium iii oxide that prevents further reaction with oxygen. The aluminum gains this charge by losing electrons during the reaction.
 Source: youtube.com
Source: youtube.com
It is also an exothermic reaction meaning that heat is released during the process. Copper ii ions will hydrolyzeto produce an excess of hydrogen ions making the copper ii chloride solution slightly acidic. 4 al s 3 o 2 g 2 al 2 o 3 s. But that isn t the whole story. How liquid metal affects copper nickel and aluminum corrosion test.
 Source: teacherspayteachers.com
Source: teacherspayteachers.com
In the aluminum half reaction the aluminum ion has a charge of positive three. The aluminum gains this charge by losing electrons during the reaction. Galvanic reaction chart below is a galvanic reaction chart for dissimilar metals. But that isn t the whole story. Like magnesium aluminium burns in oxygen with a brilliant white flame.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
Copper ii ions will hydrolyzeto produce an excess of hydrogen ions making the copper ii chloride solution slightly acidic. Aluminum metal is always covered in a thin but protective layer of aluminum oxide al 2. Aluminium reacts with oxygen forming a protective layer of alumnium iii oxide that prevents further reaction with oxygen. Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener. The aluminum gains this charge by losing electrons during the reaction.
 Source: youtube.com
Source: youtube.com
When it is reduced it gains three electrons. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. But that isn t the whole story. The aluminum gains this charge by losing electrons during the reaction. 4 al s 3 o 2 g 2 al 2 o 3 s.
 Source: uwaterloo.ca
Source: uwaterloo.ca
Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy. Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. Painting the outside of the copper pipe is recommended. This type of reaction is known as an oxidation reduction reaction or a redox reaction. Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy.
 Source: aluminumsurface.blogspot.com
Source: aluminumsurface.blogspot.com
Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. The aluminum gains this charge by losing electrons during the reaction. Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy. But that isn t the whole story. Copper ii ions will hydrolyzeto produce an excess of hydrogen ions making the copper ii chloride solution slightly acidic.
 Source: chemedx.org
Source: chemedx.org
Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy. In the aluminum half reaction the aluminum ion has a charge of positive three. Aluminium reacts with oxygen forming a protective layer of alumnium iii oxide that prevents further reaction with oxygen. Painting the outside of the copper pipe is recommended. Copper ii ions will hydrolyzeto produce an excess of hydrogen ions making the copper ii chloride solution slightly acidic.
 Source: clairebearpsp.tumblr.com
Source: clairebearpsp.tumblr.com
The product in this reaction is also alumnium iii oxide. Hot water discharge pipes should be extended beyond the roof preferably to ground. In the aluminum half reaction the aluminum ion has a charge of positive three. When it is reduced it gains three electrons. It is also an exothermic reaction meaning that heat is released during the process.
 Source: 18thtimelucky.wordpress.com
Source: 18thtimelucky.wordpress.com
In the aluminum half reaction the aluminum ion has a charge of positive three. Copper is incompatible with both zincalume steel and colorbond steel either in contact with or where water can flow from it such is as often experienced with hot water system overflows. The individual reductions or oxidations are called half reactions. Like magnesium aluminium burns in oxygen with a brilliant white flame. When it is reduced it gains three electrons.
Source: quora.com
Galvanic reaction chart below is a galvanic reaction chart for dissimilar metals. How liquid metal affects copper nickel and aluminum corrosion test. This type of reaction is known as an oxidation reduction reaction or a redox reaction. When it is reduced it gains three electrons. But that isn t the whole story.
 Source: studylib.net
Source: studylib.net
Please understand that green represents lower risk not no risk it should be noted that if sacrificial plating is incorporated in the fastener design then galvanic action can result in the deterioration of the sacrificial coating rather than of the fastener. During the reaction between copper ii chloride and aluminum the aluminum dissolves to create a solution with aluminum ions with a 3 charge and copper metal. This type of reaction is known as an oxidation reduction reaction or a redox reaction. Gallium in general also reacts with copper and leaves it pitted on the very top layer but the reaction is not heavy. 4 al s 3 o 2 g 2 al 2 o 3 s.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title does copper react with aluminum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.