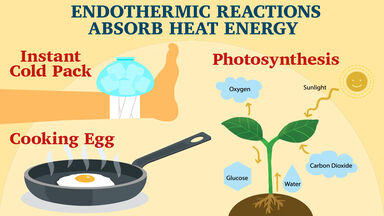
Endothermic reaction examples for kids
Endothermic Reaction Examples For Kids. Just like a bonfire keeping everyone warm. The heat energy breaks the bonds in the substance causing the reaction. As the reaction starts to slow take the temperature again. Chemical reactions are all about the energy.
 Simple Endothermic Reaction Examples From examples.yourdictionary.com
Simple Endothermic Reaction Examples From examples.yourdictionary.com
Fuseschool global education. This is actually one of the key characteristics of an endothermic reaction. Examples endothermic reaction examples. The reaction equation nh4no3 s water nh4 aq no3 aq this is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture. The heat energy breaks the bonds in the substance causing the reaction. In an endothermic reaction heat is used for the reaction to occur.
Melting of ice is also an example of an endothermic reaction.
Just like a bonfire keeping everyone warm. You may want to let the children touch the solution and compare to the room temperature water. It doesn t have to be table salt nor does the solvent need to be water. As the reaction starts to slow take the temperature again. A cold pack used in sports gets cold when the chemical inside mixes with the water bag and new bonds form causing a decrease in temperature. In an endothermic reaction heat is used for the reaction to occur.
 Source: byjus.com
Source: byjus.com
Just like a bonfire keeping everyone warm. The heat energy breaks the bonds in the substance causing the reaction. Reversible reactions are where the products can react to remake the original reactants. Endothermic reactions are reactions that need energy in the form of heat to happen or proceed. The temperature decrease can sometimes be detected using a thermometer.
 Source: slideplayer.com
Source: slideplayer.com
Exothermic reactions transfer energy to the surroundings and this energy is usually heat energy they cause the surroundings to heat up. Melting of ice is also an example of an endothermic reaction. In an endothermic reaction heat is used for the reaction to occur. As the heat is absorbed the product will be colder. Some examples of endothermic reactions are.
 Source: thoughtco.com
Source: thoughtco.com
In a sink or similar area quickly add the drink mix solution to the baking soda in the styrofoam cup. Measure 1 4 cup baking soda into the styrofoam cup. A cold pack used in sports gets cold when the chemical inside mixes with the water bag and new bonds form causing a decrease in temperature. Examples of some endothermic reactions ice melting is an example of an endothermic process not a reaction corrected this oversight as per the 11 13 12 comment below it needs heat energy to happen. It doesn t have to be table salt nor does the solvent need to be water.
 Source: thoughtco.com
Source: thoughtco.com
The reaction of barium hydroxide octahydrate crystals with dry ammonium chloride. Endothermic reactions are reactions that need energy in the form of heat to happen or proceed. Chemical reactions are all about the energy. The heat energy received by ice from its surroundings makes it melt into water. In an endothermic reaction heat is used for the reaction to occur.
 Source: thoughtco.com
Source: thoughtco.com
As the heat is absorbed the product will be colder. Endothermic reactions are reactions that need energy in the form of heat to happen or proceed. Dissolving ammonium chloride in water. Fusion of snow on a warm windshield especially for heavy snow wet. The reaction absorbs heat.
 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Measure 1 4 cup baking soda into the styrofoam cup. Fusion of snow on a warm windshield especially for heavy snow wet. The heat energy received by ice from its surroundings makes it melt into water. This is actually one of the key characteristics of an endothermic reaction. In a sink or similar area quickly add the drink mix solution to the baking soda in the styrofoam cup.
 Source: byjus.com
Source: byjus.com
Like a snowman melting. The salt dissociates into ammonium nh 4 and chloride cl ions. In a sink or similar area quickly add the drink mix solution to the baking soda in the styrofoam cup. Some examples of endothermic reactions are. The reaction of barium hydroxide octahydrate crystals with dry ammonium chloride.
 Source: slideplayer.com
Source: slideplayer.com
Reversible reactions are where the products can react to remake the original reactants. An endothermic reaction takes in energy from the surroundings. When ammonium chloride nh 4 cl is dissolved in water an endothermic reaction takes place. Just like a bonfire keeping everyone warm. Examples of some endothermic reactions ice melting is an example of an endothermic process not a reaction corrected this oversight as per the 11 13 12 comment below it needs heat energy to happen.
 Source: byjus.com
Source: byjus.com
You put heat into it. Chemical reactions are all about the energy. The heat energy received by ice from its surroundings makes it melt into water. This is actually one of the key characteristics of an endothermic reaction. Fusion of snow on a warm windshield especially for heavy snow wet.
 Source: youtube.com
Source: youtube.com
This is actually one of the key characteristics of an endothermic reaction. Exothermic reactions transfer energy to the surroundings and this energy is usually heat energy they cause the surroundings to heat up. Just like a bonfire keeping everyone warm. Fusion of snow on a warm windshield especially for heavy snow wet. The reaction of barium hydroxide octahydrate crystals with dry ammonium chloride.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
The temperature decrease can sometimes be detected using a thermometer. The temperature decrease can sometimes be detected using a thermometer. The heat energy breaks the bonds in the substance causing the reaction. Some examples of endothermic reactions are. Dissolving ammonium chloride in water.
 Source: study.com
Source: study.com
Measure 1 4 cup baking soda into the styrofoam cup. Dissolving ammonium chloride in water. Like a snowman melting. The heat energy breaks the bonds in the substance causing the reaction. As the heat is absorbed the product will be colder.
 Source: slideplayer.com
Source: slideplayer.com
The temperature decrease can sometimes be detected using a thermometer. An endothermic reaction takes in energy from the surroundings. Endothermic reactions are reactions that need energy in the form of heat to happen or proceed. When ammonium chloride nh 4 cl is dissolved in water an endothermic reaction takes place. Melting of ice is also an example of an endothermic reaction.
 Source: pinterest.com
Source: pinterest.com
Melting of ice is also an example of an endothermic reaction. When ammonium chloride nh 4 cl is dissolved in water an endothermic reaction takes place. Fusion of snow on a warm windshield especially for heavy snow wet. Just like a bonfire keeping everyone warm. The energy is usually transferred as heat energy.
 Source: sciencenotes.org
Source: sciencenotes.org
The reaction equation nh4no3 s water nh4 aq no3 aq this is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture. A cold pack used in sports gets cold when the chemical inside mixes with the water bag and new bonds form causing a decrease in temperature. Examples of some endothermic reactions ice melting is an example of an endothermic process not a reaction corrected this oversight as per the 11 13 12 comment below it needs heat energy to happen. In an endothermic reaction heat is used for the reaction to occur. You may want to let the children touch the solution and compare to the room temperature water.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title endothermic reaction examples for kids by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.