Epsom salt crystal structure
Epsom Salt Crystal Structure. Table salt or nacl forms square structures. When water is heated it can hold more magnesium sulfate. Epsom salt is another name for magnesium sulfate. Water wont boil in the microwave it will make a mess.
 About Epsom Salt From epsom-salts.weebly.com
About Epsom Salt From epsom-salts.weebly.com
As a hydrate epsom salt is considered a monoclinic crystal structure. However when the mixture is refrigerated the magnesium sulfate atoms pull away from the water and form a crystal structure because the water can no longer hold all of the epsom salt. When you cool the solution very fast the conditions are unstable. The water will have more salt than it can hold it is supersaturated. Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. Because in a microwave the water is heated past its boiling point before it has.
Because in a microwave the water is heated past its boiling point before it has.
When you heat water and stir in epsom salt the water absorbs the salt. Sponge optional food coloring optional. Shallow bowl or dish. Table salt or nacl forms square structures. When you cool the solution very fast the conditions are unstable. 1 4 cup epsom salts magnesium sulfate 1 2 cup water.
 Source: curiouschemeng.blogspot.com
Source: curiouschemeng.blogspot.com
Epsom salt is another name for magnesium sulfate. As your salt solution cools down the magnesium sulfate atoms run into each other and form the crystal. However when the mixture is refrigerated the magnesium sulfate atoms pull away from the water and form a crystal structure because the water can no longer hold all of the epsom salt. As a hydrate epsom salt is considered a monoclinic crystal structure. 11 years ago on introduction.
 Source: thoughtco.com
Source: thoughtco.com
However when the mixture is refrigerated the magnesium sulfate atoms pull away from the water and form a crystal structure because the water can no longer hold all of the epsom salt. Due to the nature of its elements and their bonds table salt will not form crystals such as these. Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. 1 4 cup epsom salts magnesium sulfate 1 2 cup water. This means it has three axes where two intersect and are then perpendicular to the third one as shown in the picture below.
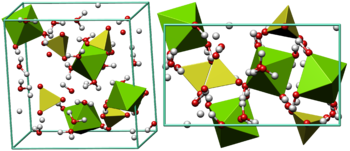 Source: en.wikipedia.org
Source: en.wikipedia.org
As a hydrate epsom salt is considered a monoclinic crystal structure. When you heat water and stir in epsom salt the water absorbs the salt. As your salt solution cools down the magnesium sulfate atoms run into each other and form the crystal. Water wont boil in the microwave it will make a mess. 11 years ago on introduction.
 Source: thoughtco.com
Source: thoughtco.com
Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. When you cool the solution very fast the conditions are unstable. However when the mixture is refrigerated the magnesium sulfate atoms pull away from the water and form a crystal structure because the water can no longer hold all of the epsom salt. Epsom salt is another name for magnesium sulfate. Due to the nature of its elements and their bonds table salt will not form crystals such as these.
 Source: curiouschemeng.blogspot.com
Source: curiouschemeng.blogspot.com
Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. When you cool the solution very fast the conditions are unstable. Shallow bowl or dish. Table salt or nacl forms square structures. Water wont boil in the microwave it will make a mess.
 Source: ingridscience.ca
Source: ingridscience.ca
Due to the nature of its elements and their bonds table salt will not form crystals such as these. Sponge optional food coloring optional. Shallow bowl or dish. Because in a microwave the water is heated past its boiling point before it has. Water wont boil in the microwave it will make a mess.
 Source: epsom-salts.weebly.com
Source: epsom-salts.weebly.com
When you cool the solution very fast the conditions are unstable. Sponge optional food coloring optional. Epsom salt crystal materials. When you heat water and stir in epsom salt the water absorbs the salt. As a hydrate epsom salt is considered a monoclinic crystal structure.
 Source: pinterest.com
Source: pinterest.com
The water will have more salt than it can hold it is supersaturated. When you heat water and stir in epsom salt the water absorbs the salt. 11 years ago on introduction. The water will have more salt than it can hold it is supersaturated. Table salt or nacl forms square structures.
 Source: pinterest.com
Source: pinterest.com
11 years ago on introduction. Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. 1 4 cup epsom salts magnesium sulfate 1 2 cup water. Shallow bowl or dish. Table salt or nacl forms square structures.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. Epsom salt crystal materials. When you heat water and stir in epsom salt the water absorbs the salt. Epsom salt is another name for magnesium sulfate. Sponge optional food coloring optional.
 Source: curiouschemeng.blogspot.com
Source: curiouschemeng.blogspot.com
Shallow bowl or dish. Water wont boil in the microwave it will make a mess. When water is heated it can hold more magnesium sulfate. Table salt or nacl forms square structures. This means it has three axes where two intersect and are then perpendicular to the third one as shown in the picture below.
 Source: ingridscience.ca
Source: ingridscience.ca
11 years ago on introduction. Water wont boil in the microwave it will make a mess. This means it has three axes where two intersect and are then perpendicular to the third one as shown in the picture below. Due to the nature of its elements and their bonds table salt will not form crystals such as these. 1 4 cup epsom salts magnesium sulfate 1 2 cup water.
 Source: babbledabbledo.com
Source: babbledabbledo.com
Due to the nature of its elements and their bonds table salt will not form crystals such as these. However when the mixture is refrigerated the magnesium sulfate atoms pull away from the water and form a crystal structure because the water can no longer hold all of the epsom salt. Epsom salt crystal materials. This means it has three axes where two intersect and are then perpendicular to the third one as shown in the picture below. 1 4 cup epsom salts magnesium sulfate 1 2 cup water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The water will have more salt than it can hold it is supersaturated. The water will have more salt than it can hold it is supersaturated. Epsom salt is the regular name for magnesium sulfate. 11 years ago on introduction. When water is heated it can hold more magnesium sulfate.
 Source: scienceprojectideas.org
Source: scienceprojectideas.org
Epsom salt is the regular name for magnesium sulfate. As your salt solution cools down the magnesium sulfate atoms run into each other and form the crystal. Epsom salt is the regular name for magnesium sulfate. Epsom salt looks similar to normal table salt in that is solid odorless small and white clear but it s crystal size is usually a bit bigger. Shallow bowl or dish.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title epsom salt crystal structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







