Examples of elements compounds and mixtures
Examples Of Elements Compounds And Mixtures. Examples of mixtures are sea water air and dirt. Chemical substances are often called pure to set them apart from mixtures. Mixtures can usually be separated by physical techniques such as filtering and. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together.
 Examplesof Net Example Of Elements Compounds And Mixtures From examplesof.net
Examplesof Net Example Of Elements Compounds And Mixtures From examplesof.net
Mixtures are composed of variable proportions of molecules and atoms. Copper cu oxygen o hydrogen h helium he lithium li b compound. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. Mixtures can be a solid liquid or a gas.
A compound is a substance which contains two or more elements chemically combined together.
Water h 2 o table salt nacl. Mixtures can be a solid liquid or a gas. Carbon dioxide is up of carbon and oxygen. Air the cytosol of a cell examples of compounds. Examples of mixtures are sea water air and dirt. Chemical substances are often called pure to set them apart from mixtures.
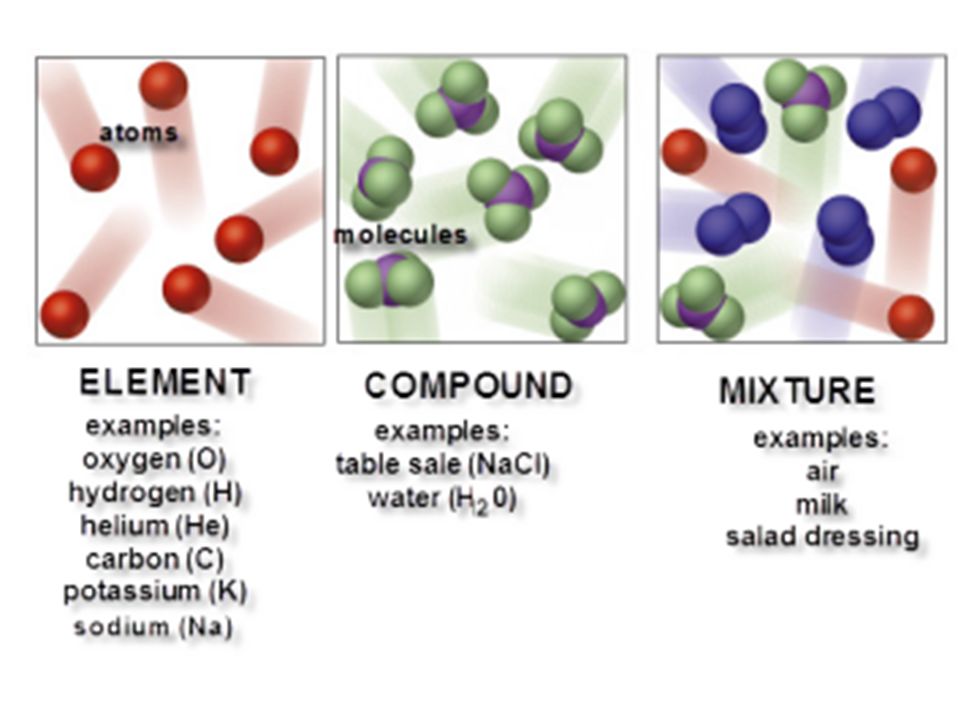
Examples of mixtures are sea water air and dirt. Mixtures can usually be separated by physical techniques such as filtering and. Compounds containing bonds between carbon and a metal are called organometallic compounds. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. Mixture of an element that exists in the form of molecules and a compound.
 Source: sciencewitheberhart.weebly.com
Source: sciencewitheberhart.weebly.com
Mixture of two elements both of which exist as molecules rather than atoms. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together. Water is made up of hydrogen and oxygen atoms. A compound is a substance which contains two or more elements chemically combined together. Air the cytosol of a cell examples of compounds.
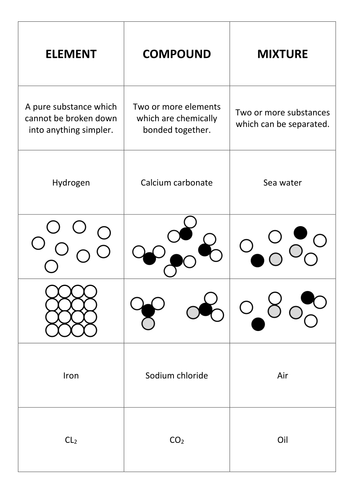
Another difference between compounds and mixtures of elements is the ease with which the elements can be separated. Compounds containing bonds between carbon and a metal are called organometallic compounds. Air the cytosol of a cell examples of compounds. Copper cu oxygen o hydrogen h helium he lithium li b compound. Mixtures can usually be separated by physical techniques such as filtering and.
 Source: pinterest.com
Source: pinterest.com
Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Examples of mixtures are sea water air and dirt. Water is made up of hydrogen and oxygen atoms. Chemical substances are often called pure to set them apart from mixtures. Compounds made primarily of carbon and hydrogen atoms are called organic compounds and all others are called inorganic compounds.
 Source: sites.google.com
Source: sites.google.com
Example of compounds includes water h 2 o hydrogen peroxide h 2 o 2 etc. Examples of mixtures are sea water air and dirt. Water is made up of hydrogen and oxygen atoms. Mixture of an element that exists in the form of molecules and a compound. It can contain as little as 10 or as much as 45 zinc.
 Source: pinterest.com
Source: pinterest.com
Compounds are homogeneous forms of matter. Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Another difference between compounds and mixtures of elements is the ease with which the elements can be separated. Air the cytosol of a cell examples of compounds. Examples of mixtures are sea water air and dirt.
 Source: aplustopper.com
Source: aplustopper.com
An impure substance made from different elements or compounds mixed together that are not chemically joined. Examples of mixtures are sea water air and dirt. Mixtures can be a solid liquid or a gas. Chemical substances are often called pure to set them apart from mixtures. Mixtures such as the atmosphere contain two or more substances that are relatively easy to separate.
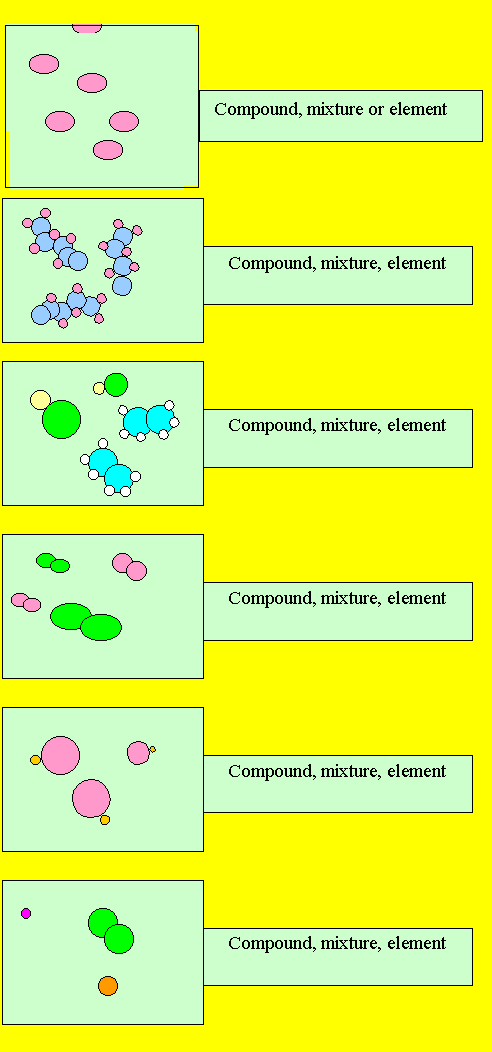 Source: dynamicscience.com.au
Source: dynamicscience.com.au
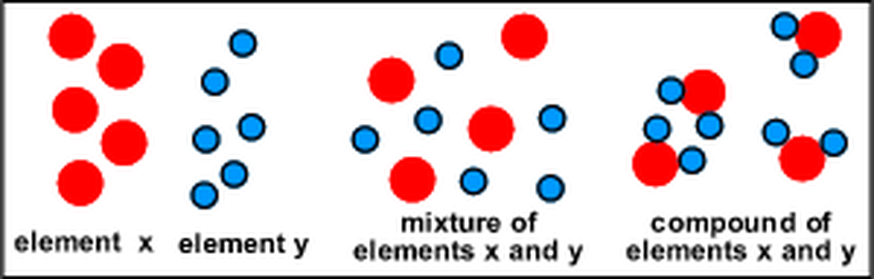
Mixtures are composed of variable proportions of molecules and atoms. Mixture of two elements one of which exists as atoms the other as molecules. While compounds and mixtures can be separated through a variety of techniques elements cannot as they exist in the purest form possible. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. A compound is a substance which contains two or more elements chemically combined together.
 Source: 123rf.com
Source: 123rf.com
Mixture of an element that exists in the form of molecules and a compound. Compounds containing bonds between carbon and a metal are called organometallic compounds. Brass is an example of a mixture of two elements. Mixtures are composed of variable proportions of molecules and atoms. Air the cytosol of a cell examples of compounds.

Chemical substances are often called pure to set them apart from mixtures. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. It can contain as little as 10 or as much as 45 zinc. Mixtures are composed of variable proportions of molecules and atoms. Compounds are homogeneous forms of matter.
 Source: examplesof.net
Source: examplesof.net
Brass is an example of a mixture of two elements. They are more easily separated than compounds. Example of compounds includes water h 2 o hydrogen peroxide h 2 o 2 etc. Compounds containing bonds between carbon and a metal are called organometallic compounds. Water is made up of hydrogen and oxygen atoms.
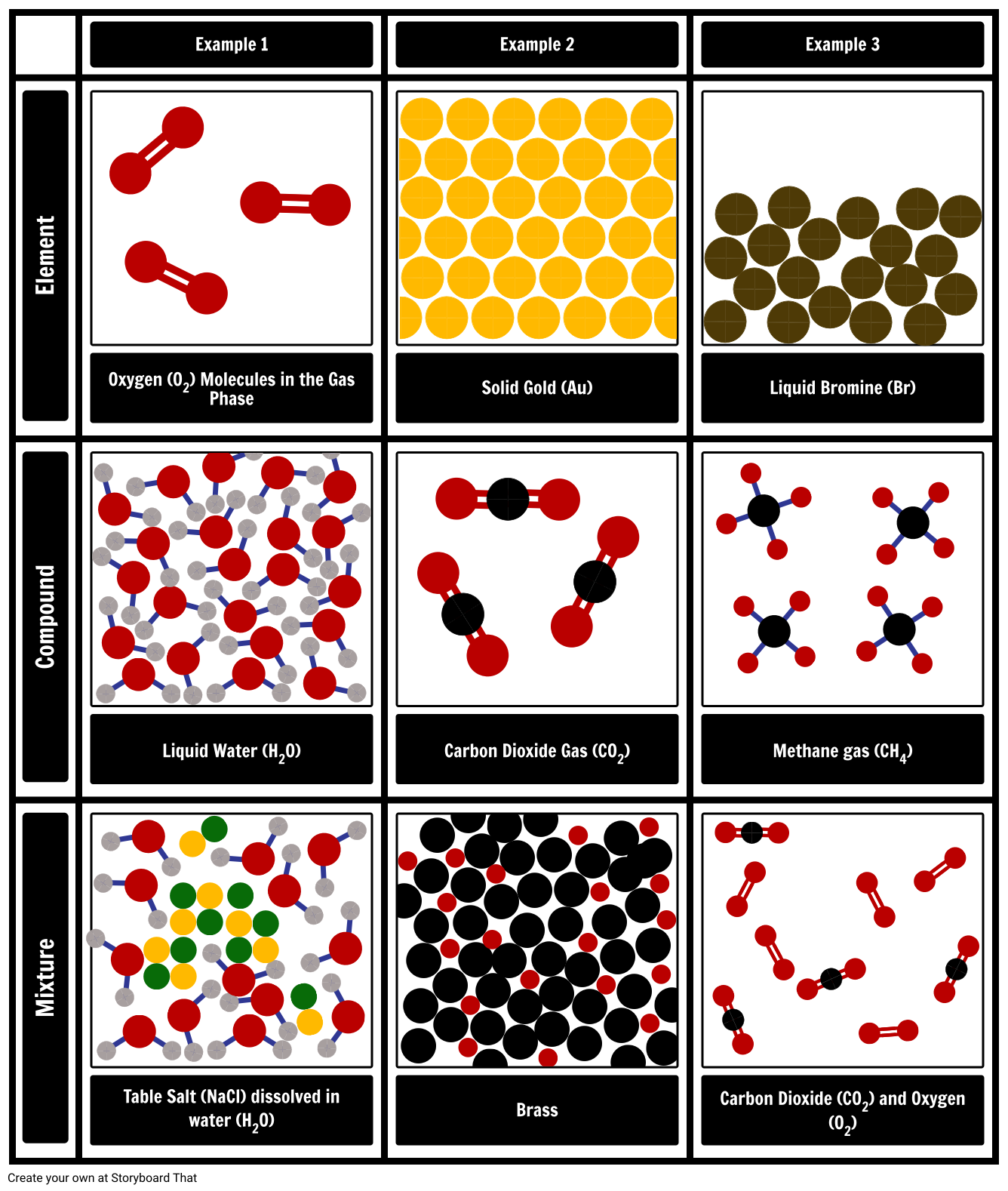 Source: storyboardthat.com
Source: storyboardthat.com
Soil ocean water and other solutions. Air the cytosol of a cell examples of compounds. Soil ocean water and other solutions. Chemical substances are often called pure to set them apart from mixtures. Mixtures can be a solid liquid or a gas.
 Source: proprofs.com
Source: proprofs.com
Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together. Brass is an example of a mixture of two elements. While compounds and mixtures can be separated through a variety of techniques elements cannot as they exist in the purest form possible. Mixture of two elements both of which exist as molecules rather than atoms. A compound is a substance which contains two or more elements chemically combined together.
 Source: slideshare.net
Source: slideshare.net
A compound is a substance which contains two or more elements chemically combined together. Mixture of an element that exists in the form of molecules and a compound. Compounds are homogeneous forms of matter. Chemical substances are often called pure to set them apart from mixtures. It can contain as little as 10 or as much as 45 zinc.
 Source: storyboardthat.com
Source: storyboardthat.com
They are more easily separated than compounds. An impure substance made from different elements or compounds mixed together that are not chemically joined. Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Mixtures can be a solid liquid or a gas. You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title examples of elements compounds and mixtures by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.