Examples of metals on the periodic table
Examples Of Metals On The Periodic Table. They are soft metals that are highly reactive and have one electron in their outermost s sub shell. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. A metal s use is directly linked to its qualities. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them.
 List Of Metals From sciencenotes.org
List Of Metals From sciencenotes.org
Most elements are metals. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. Other examples of pure metal include californium copper europium radium cadmium einsteinium titanium and tungsten. They are soft metals that are highly reactive and have one electron in their outermost s sub shell. Most elements can be considered metals.
Transition metals are those metals in groups three through 12 on the periodic table.
Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks. Other examples of pure metal include californium copper europium radium cadmium einsteinium titanium and tungsten. Shiny metals such as copper silver and gold are often used for decorative arts jewelry and coins. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks. In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons.
 Source: socratic.org
Source: socratic.org
The six alkali metals are. Transition metals are those metals in groups three through 12 on the periodic table. Alloys such as brass and bronze also are metals. Metals exhibit the following properties. The six alkali metals are.
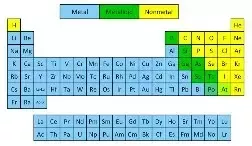 Source: quora.com
Source: quora.com
The alkali metals can be found in the first column on the left side of the periodic table. Examples of metals most of the elements on the periodic table are metals including gold silver platinum mercury uranium aluminum sodium and calcium. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. The six alkali metals are.
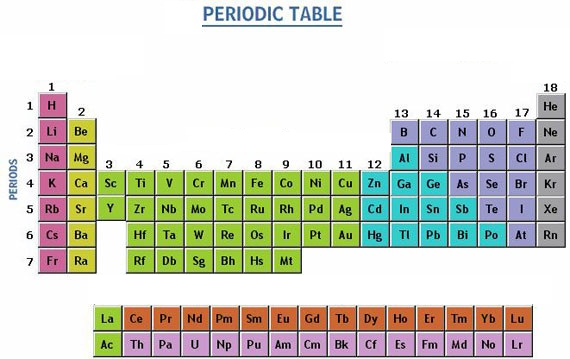 Source: periodictable.me
Source: periodictable.me
Other examples of pure metal include californium copper europium radium cadmium einsteinium titanium and tungsten. Examples of metals include iron tin sodium and plutonium. The alkali metals can be found in the first column on the left side of the periodic table. They are grouped together in the middle to the left hand side of the periodic table. The highlighted elements are considered the metal elements.
 Source: sciencenotes.org
Source: sciencenotes.org
They are soft metals that are highly reactive and have one electron in their outermost s sub shell. Metals exhibit the following properties. Most elements can be considered metals. The six alkali metals are. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks.
 Source: slideshare.net
Source: slideshare.net
Examples of metals most of the elements on the periodic table are metals including gold silver platinum mercury uranium aluminum sodium and calcium. Shiny metals such as copper silver and gold are often used for decorative arts jewelry and coins. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. Other examples of pure metal include californium copper europium radium cadmium einsteinium titanium and tungsten. Transition metals are those metals in groups three through 12 on the periodic table.
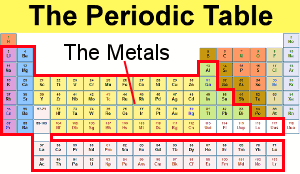 Source: chemicool.com
Source: chemicool.com
Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks. Examples of metals include iron tin sodium and plutonium. A metal s use is directly linked to its qualities. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po.
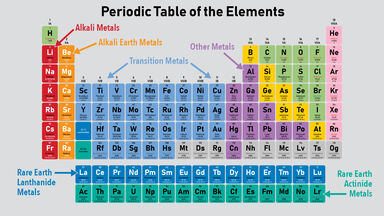 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Metals exhibit the following properties. Alloys such as brass and bronze also are metals. The alkali metals can be found in the first column on the left side of the periodic table. Other examples of pure metal include californium copper europium radium cadmium einsteinium titanium and tungsten. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks.
Source: biology.ualberta.ca
Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. Examples of metals include iron tin sodium and plutonium. Usually solid at room temperature mercury is an exception. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks.
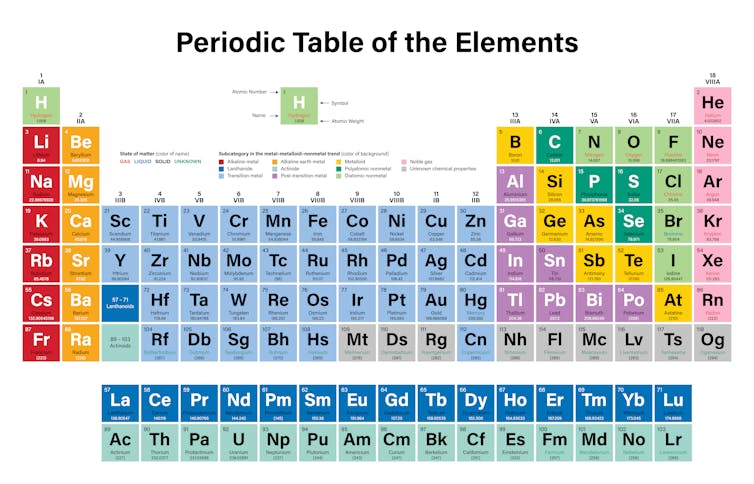 Source: theconversation.com
Source: theconversation.com
Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. Most elements are metals. They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals.
 Source: pinterest.com
Source: pinterest.com
In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals. Examples of metals most of the elements on the periodic table are metals including gold silver platinum mercury uranium aluminum sodium and calcium. Transition metals are those metals in groups three through 12 on the periodic table. Most elements are metals.
 Source: sciencenotes.org
Source: sciencenotes.org
The highlighted elements are considered the metal elements. Examples of metals include iron tin sodium and plutonium. Usually solid at room temperature mercury is an exception. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals.
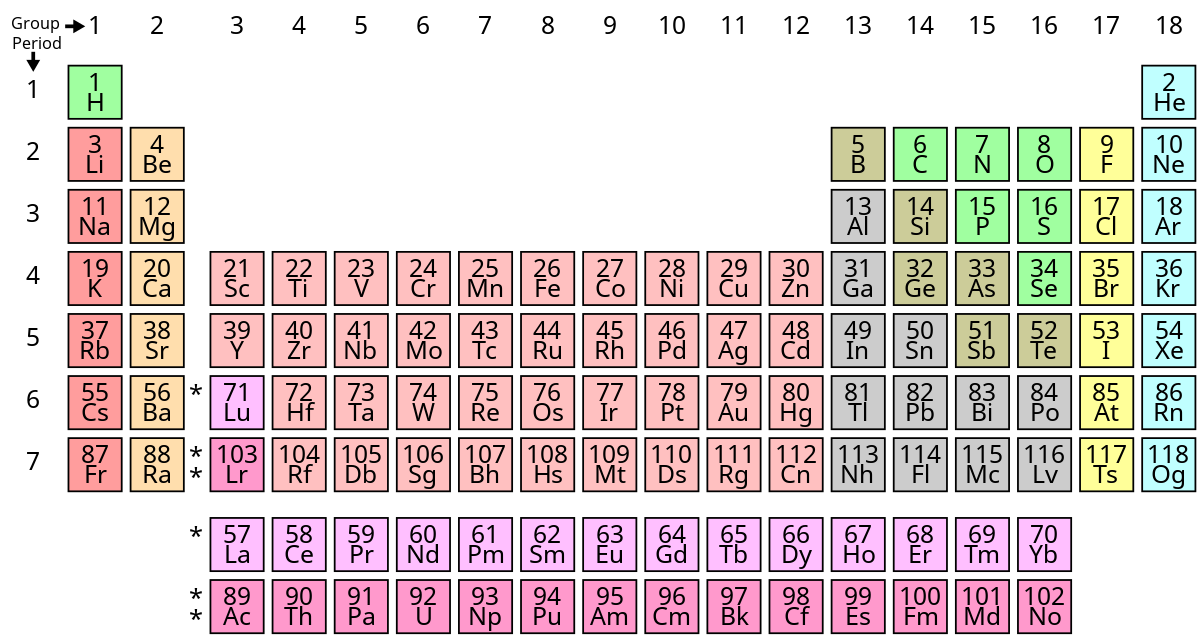 Source: en.wikipedia.org
Source: en.wikipedia.org
The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. They are soft metals that are highly reactive and have one electron in their outermost s sub shell. They are grouped together in the middle to the left hand side of the periodic table. Most elements are metals. Strong metals such as iron and metal alloys such as stainless steel are used to build structures ships and vehicles including cars trains and trucks.
Source: researchgate.net
They have an incomplete inner electron shell and they serve as links between those metals that have the most and least electrons. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals. Most elements are metals. A metal s use is directly linked to its qualities.
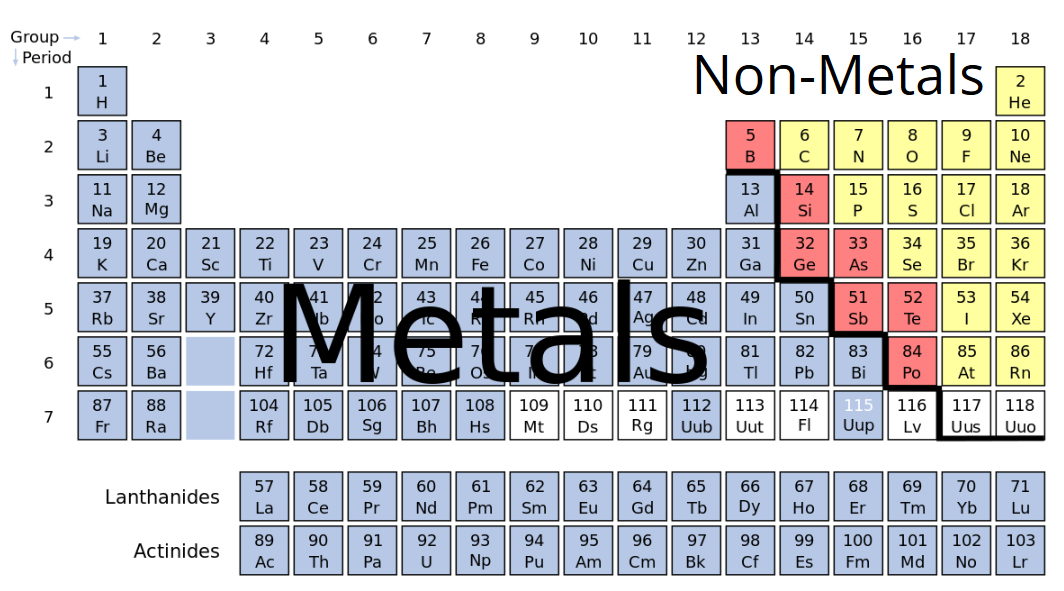 Source: sciencetrends.com
Source: sciencetrends.com
Alloys such as brass and bronze also are metals. They are soft metals that are highly reactive and have one electron in their outermost s sub shell. Usually solid at room temperature mercury is an exception. Most elements can be considered metals. The highlighted elements are considered the metal elements.
 Source: thoughtco.com
Source: thoughtco.com
Most elements can be considered metals. Usually solid at room temperature mercury is an exception. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Most elements can be considered metals. The alkali metals can be found in the first column on the left side of the periodic table.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title examples of metals on the periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







