Experiment elephant toothpaste
Experiment Elephant Toothpaste. The experiment is also known as the marshmallow experiment but is unrelated to the p. This is the same as a 12 solution liquid dish soap. You can add 5 grams of starch to the hydrogen peroxide. Package of dry yeast found at the grocery store measuring spoons.
 The Elephant S Toothpaste Experiment Sciencebob Com From sciencebob.com
The Elephant S Toothpaste Experiment Sciencebob Com From sciencebob.com
Variations of the elephant toothpaste demonstration. Package of dry yeast found at the grocery store measuring spoons. The movement of the. You can use yeast instead of potassium iodide. You ll see here that we have two bottles. Hydrogen peroxide h 2 o 2 decomposes into water and oxygen gas which is in the form of foam but normally the.
The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain.
Hydrogen peroxide 12 this is found at a store that sells hair care products. The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain. It is the result of a chemical reaction that creates a large amount of oozing foam. A bubbly science project from science buddies. Hydrogen peroxide h 2 o 2 decomposes into water and oxygen gas which is in the form of foam but normally the. Package of dry yeast found at the grocery store measuring spoons.
 Source: preschoolpowolpackets.blogspot.com
Source: preschoolpowolpackets.blogspot.com
By science buddies ben finio on august 1 2019. أعرض هذا باللغة العربية. How rapidly the reaction proceeds will depend on the concentration of hydrogen peroxide. A clean 16 oz plastic soda bottle. The elephant toothpaste reaction is just the speeding up of a chemical reaction that usually happens very slowly.
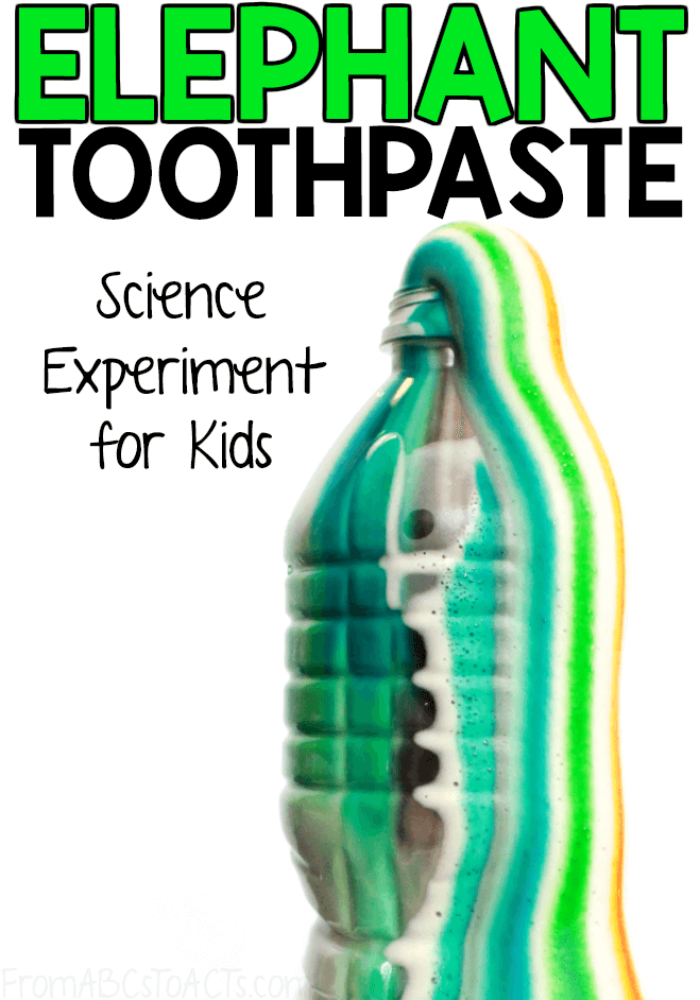 Source: fromabcstoacts.com
Source: fromabcstoacts.com
Why does the elephant s toothpaste happen. The chemical formula for hydrogen peroxide is h 2 o 2 which. Hydrogen peroxide h 2 o 2 decomposes into water and oxygen gas which is in the form of foam but normally the. 1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution. Elephant toothpaste experiment instructions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is the result of a chemical reaction that creates a large amount of oozing foam. You can get this from a beauty supply store or hair salon. The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain. It is the result of a chemical reaction that creates a large amount of oozing foam. You can use yeast instead of potassium iodide.

The foam your kids will create in this experiment resembles toothpaste being squeezed from a tube just be sure they don t get it in their mouth. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium permangamate or potassium iodide as a catalysts. The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain. You ll see here that we have two bottles. This experiment is sometimes called elephant s toothpaste because it looks like toothpaste coming out of a tube but don t get the foam in your mouth.
 Source: funwithmama.com
Source: funwithmama.com
Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium permangamate or potassium iodide as a catalysts. The elephant toothpaste reaction is just the speeding up of a chemical reaction that usually happens very slowly. The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain. This experiment is sometimes called elephant s toothpaste because it looks like toothpaste coming out of a tube but don t get the foam in your mouth. Hydrogen peroxide that antiseptic liquid that usually comes in a brown bottle and bubbles up when you put it on a cut is a chemical compound that s made of two hydrogen and two oxygen molecules bonded together.
 Source: youtube.com
Source: youtube.com
Ask for hydrogen peroxide that is labeled 40 volume. The foam produced is just water soap and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium iodide or yeast and warm water as a catalyst. أعرض هذا باللغة العربية. 1 liter plastic soda bottle.
 Source: hellowonderful.co
Source: hellowonderful.co
The elephant toothpaste reaction is just the speeding up of a chemical reaction that usually happens very slowly. The movement of the. 1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium iodide or yeast and warm water as a catalyst. A clean 16 oz plastic soda bottle.
 Source: sciencebob.com
Source: sciencebob.com
It is the result of a chemical reaction that creates a large amount of oozing foam. Hydrogen peroxide 12 this is found at a store that sells hair care products. When the potassium iodide is added the resulting foam will have light and dark patches from the reaction of some of the starch to form triiodide. The foam your kids will create in this experiment resembles toothpaste being squeezed from a tube just be sure they don t get it in their mouth. The movement of the.
 Source: babbledabbledo.com
Source: babbledabbledo.com
You can use yeast instead of potassium iodide. Hydrogen peroxide h 2 o 2 decomposes into water and oxygen gas which is in the form of foam but normally the. How rapidly the reaction proceeds will depend on the concentration of hydrogen peroxide. Variations of the elephant toothpaste demonstration. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium iodide or yeast and warm water as a catalyst.
 Source: wcax.com
Source: wcax.com
Because it requires only a small number of ingredients and makes a volcano of foam this is a popular experiment for children to perform in school or at parties. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium permangamate or potassium iodide as a catalysts. The foam your kids will create in this experiment resembles toothpaste being squeezed from a tube just be sure they don t get it in their mouth. Elephant toothpaste experiment instructions. Variations of the elephant toothpaste demonstration.
 Source: coolscienceexperimentshq.com
Source: coolscienceexperimentshq.com
1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution. 1 liter plastic soda bottle. You can get this from a beauty supply store or hair salon. The experiment is also known as the marshmallow experiment but is unrelated to the p. The foam your kids will create in this experiment resembles toothpaste being squeezed from a tube just be sure they don t get it in their mouth.
 Source: thoughtco.com
Source: thoughtco.com
When the potassium iodide is added the resulting foam will have light and dark patches from the reaction of some of the starch to form triiodide. How rapidly the reaction proceeds will depend on the concentration of hydrogen peroxide. Elephant s toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide using potassium permangamate or potassium iodide as a catalysts. Ask for hydrogen peroxide that is labeled 40 volume. 1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution.
 Source: safety.com
Source: safety.com
Hydrogen peroxide h 2 o 2 decomposes into water and oxygen gas which is in the form of foam but normally the. Making elephant toothpaste is an easy and fun science experiment that you can do with your kids at home or with students in the lab. Hydrogen peroxide that antiseptic liquid that usually comes in a brown bottle and bubbles up when you put it on a cut is a chemical compound that s made of two hydrogen and two oxygen molecules bonded together. It is the result of a chemical reaction that creates a large amount of oozing foam. 1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution.
 Source: teachbesideme.com
Source: teachbesideme.com
This experiment shows the catalyzed decomposition of hydrogen peroxide. A clean 16 oz plastic soda bottle. When the potassium iodide is added the resulting foam will have light and dark patches from the reaction of some of the starch to form triiodide. 1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution. You can add 5 grams of starch to the hydrogen peroxide.
 Source: sciencebob.com
Source: sciencebob.com
1 2 cup 20 volume hydrogen peroxide liquid 20 volume is a 6 solution. The movement of the. Making elephant toothpaste is an easy and fun science experiment that you can do with your kids at home or with students in the lab. 1 liter plastic soda bottle. Step 1 combine two tablespoons of warm water with one teaspoon of yeast and mix until the yeast is completely dissolved in the water.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title experiment elephant toothpaste by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.