Finding molarity of a solution
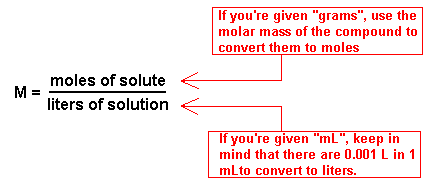
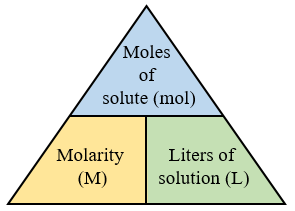
Finding Molarity Of A Solution. Molarity moles solute liter solution. Molarity moles of solute liters of solution 0 09 mol 0 8 l 0 1125 mol l. Liters of solution ml of solution x 1 l 1000 ml liters of solution 750 ml x 1 l 1000 ml liters of solution 0 75 l. N is the number of moles of the solute v is the volume of solution given in terms of litres.
 Calculate The Molarity Of 0 950 Mol Of Na2 Clutch Prep From clutchprep.com
Calculate The Molarity Of 0 950 Mol Of Na2 Clutch Prep From clutchprep.com
What other calculations you can do with the molarity calculator. Liters of solution ml of solution x 1 l 1000 ml liters of solution 750 ml x 1 l 1000 ml liters of solution 0 75 l. Expressed the weight of a solute measured in grams needed to be dissolved in a specific volume of solution to obtain the required molar concentration. To calculate molarity you may have to use conversion factors to move between units. How molarity is used to quantify the concentration of solute and calculations related to molarity. Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9.
This is enough to calculate the molarity.
The equation for calculating molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute. Mass g concentration mol l x volume l x molecular weight g mol volume of a solution calculation. Molar concentration or molarity mol liter mass volume x 1 molecular weight number of moles of solute volume of solution where the mass. This example is prepared with enough water to make 750 ml of solution. Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9. Atomic mass of cl 35 45.
 Source: socratic.org
Source: socratic.org
Molarity moles of solute liters of solution 0 09 mol 0 8 l 0 1125 mol l. To find the molarity of the ions first determine the molarity of the solute and the ion to solute ratio. In order to find the molarity you need to divide 0 09 mol the number of moles of the solute nacl by 0 8 l the volume of the solution in liters. Mass of a compound of a solution calculation. The equation for calculating molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Molarity moles of solute liters of solution 0 09 mol 0 8 l 0 1125 mol l. What other calculations you can do with the molarity calculator. Expressed the weight of a solute measured in grams needed to be dissolved in a specific volume of solution to obtain the required molar concentration. Mass g concentration mol l x volume l x molecular weight g mol volume of a solution calculation. M frac n v here m is the molality of the solution that is to be calculated.
 Source: researchgate.net
Source: researchgate.net
From the periodic table. How molarity is used to quantify the concentration of solute and calculations related to molarity. From the periodic table. Divide the number of moles of solute by the number of liters of solution. Find the molarity of the solute.
 Source: youtube.com
Source: youtube.com
Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9. The equation for calculating molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute. Mass of a compound of a solution calculation. To calculate molarity you may have to use conversion factors to move between units. In order to find the molarity you need to divide 0 09 mol the number of moles of the solute nacl by 0 8 l the volume of the solution in liters.
 Source: wikihow.com
Source: wikihow.com
How molarity is used to quantify the concentration of solute and calculations related to molarity. In order to find the molarity you need to divide 0 09 mol the number of moles of the solute nacl by 0 8 l the volume of the solution in liters. Mass g concentration mol l x volume l x molecular weight g mol volume of a solution calculation. M frac n v here m is the molality of the solution that is to be calculated. Molarity or molar concentration is the number of moles of solute per liter of solution measured in mol liter denoted as m and calculated as follows where number of moles of solute liters of solution.
 Source: thoughtco.com
Source: thoughtco.com
Molarity moles solute liter solution. Molarity moles solute liter solution. This example is prepared with enough water to make 750 ml of solution. Atomic mass of cl 35 45. Definitions of solution solute and solvent.
 Source: chegg.com
Source: chegg.com
Definitions of solution solute and solvent. Mass of a compound of a solution calculation. This is enough to calculate the molarity. In order to find the molarity you need to divide 0 09 mol the number of moles of the solute nacl by 0 8 l the volume of the solution in liters. Molar concentration or molarity mol liter mass volume x 1 molecular weight number of moles of solute volume of solution where the mass.
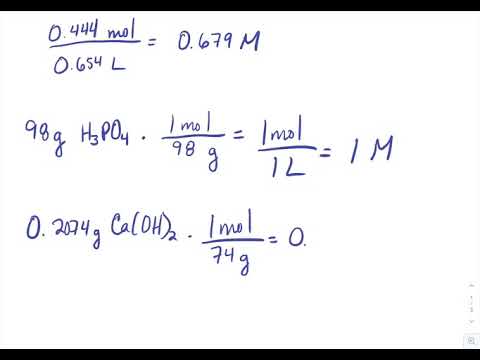 Source: chem.libretexts.org
Source: chem.libretexts.org
Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9. Volume l mass g concentration mol l x molecular weight g mol molecular weight of a solvent in a solution calculation. Mass g concentration mol l x volume l x molecular weight g mol volume of a solution calculation. Divide the number of moles of solute by the number of liters of solution. Atomic mass of cu 63 55.
 Source: timesoracle.com
Source: timesoracle.com
Molarity moles of solute liters of solution 0 09 mol 0 8 l 0 1125 mol l. Liters of solution ml of solution x 1 l 1000 ml liters of solution 750 ml x 1 l 1000 ml liters of solution 0 75 l. Definitions of solution solute and solvent. What other calculations you can do with the molarity calculator. For example if you re given the mass of a solute in grams use the molar mass usually rounded to two decimal places of that solute to convert the given mass into moles.
 Source: m.youtube.com
Source: m.youtube.com
Atomic mass of cu 63 55. Convert 750 ml to liters. From the periodic table. To find the molarity of the ions first determine the molarity of the solute and the ion to solute ratio. Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Convert 750 ml to liters. Atomic mass of cu 63 55. Convert 750 ml to liters. How molarity is used to quantify the concentration of solute and calculations related to molarity. Atomic mass of cl 35 45.
 Source: researchgate.net
Source: researchgate.net
Atomic mass of cucl 2 1 63 55 2 35 45 atomic mass of cucl 2 63 55 70 9. Molarity or molar concentration is the number of moles of solute per liter of solution measured in mol liter denoted as m and calculated as follows where number of moles of solute liters of solution. N is the number of moles of the solute v is the volume of solution given in terms of litres. In order to find the molarity you need to divide 0 09 mol the number of moles of the solute nacl by 0 8 l the volume of the solution in liters. For example if you re given the mass of a solute in grams use the molar mass usually rounded to two decimal places of that solute to convert the given mass into moles.
 Source: clutchprep.com
Source: clutchprep.com
M frac n v here m is the molality of the solution that is to be calculated. Atomic mass of cu 63 55. N is the number of moles of the solute v is the volume of solution given in terms of litres. Find the molarity of the solute. Definitions of solution solute and solvent.
 Source: youtube.com
Source: youtube.com
To find the molarity of the ions first determine the molarity of the solute and the ion to solute ratio. Find the molarity of the solute. Atomic mass of cl 35 45. The equation for calculating molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute. This is enough to calculate the molarity.
 Source: pinterest.com
Source: pinterest.com
Find the molarity of the solute. What other calculations you can do with the molarity calculator. Molarity relates the amount of solute to the volume of the solution. Molar concentration or molarity mol liter mass volume x 1 molecular weight number of moles of solute volume of solution where the mass. M frac n v here m is the molality of the solution that is to be calculated.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title finding molarity of a solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.