Flame test elements
Flame Test Elements. Colored flames from strontium cesium sodium and lithium from left to right. All of my results matched with the expected colors listed in the background. As well as copper and lead and barium and manganese. Small glass dish for each powder tested.

As well as copper and lead and barium and manganese. Small cup of water. For other metals there are usually other easy methods which are more reliable but the flame test can give a useful hint as to where to look. For group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. About press copyright contact us creators advertise developers terms privacy policy safety how youtube works. Colored flames from strontium cesium sodium and lithium from left to right.
For example copper i emits blue light during the flame test while copper ii emits green light.
Carrying out a flame test. It is widely used to detect and analyze the presence of certain elements in the given salt or compound. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Different elements produce distinct colors in the flame test because it is the result of the different valence electrons in their specific orbitals. Carrying out a flame test. All of my results matched with the expected colors listed in the background.
 Source: pinterest.com
Source: pinterest.com
All of my results matched with the expected colors listed in the background. Container of water to douse the flame. Not all metal ions give flame colours. Put the loop into the edge of the blue flame from a bunsen burner. About press copyright contact us creators advertise developers terms privacy policy safety how youtube works.
 Source: open.edu
Source: open.edu
Different elements produce distinct colors in the flame test because it is the result of the different valence electrons in their specific orbitals. Put the loop into the edge of the blue flame from a bunsen burner. For group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. The flame test is relatively quick and simple to perform and can be carried out with the basic equipment found in most chemistry laboratories. Different elements produce distinct colors in the flame test because it is the result of the different valence electrons in their specific orbitals.
 Source: thoughtco.com
Source: thoughtco.com
Small glass dish for each powder tested. About press copyright contact us creators advertise developers terms privacy policy safety how youtube works. For other metals there are usually other easy methods that are more reliable but the flame test can give a useful hint as to where to look. To carry out a flame test. For group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got.
 Source: dornsife.usc.edu
Source: dornsife.usc.edu
Small glass dish for each powder tested. Colored flames from strontium cesium sodium and lithium from left to right. Put the loop into the edge of the blue flame from a bunsen burner. For example copper i emits blue light during the flame test while copper ii emits green light. Here are a couple of household materials that contain metals that are easily seen and identifiable in a flame test.
 Source: sciencenotes.org
Source: sciencenotes.org
Colored flames from strontium cesium sodium and lithium from left to right. As well as copper and lead and barium and manganese. Carrying out a flame test. The flame test can be used to distinguish between the oxidation states of atoms of a single element too. Li na k ca s.
 Source: study.com
Source: study.com
For example copper i emits blue light during the flame test while copper ii emits green light. For other metals there are usually other easy methods which are more reliable but the flame test can give a useful hint as to where to look. A metal salt consists of a component cation the metal and an anion. All of my results matched with the expected colors listed in the background. The flame test is used to visually determine the identity of an unknown metal of an ionic salt based on the.
 Source: buzzfeednews.com
Source: buzzfeednews.com
It is widely used to detect and analyze the presence of certain elements in the given salt or compound. The flame test is used to visually determine the identity of an unknown metal of an ionic salt based on the. For other metals there are usually other easy methods which are more reliable but the flame test can give a useful hint as to where to look. As well as copper and lead and barium and manganese. Container of water to douse the flame.
 Source: thoughtco.com
Source: thoughtco.com
Carrying out a flame test. It is widely used to detect and analyze the presence of certain elements in the given salt or compound. Small cup of water. For other metals there are usually other easy methods which are more reliable but the flame test can give a useful hint as to where to look. All of my results matched with the expected colors listed in the background.
 Source: chem.libretexts.org
Source: chem.libretexts.org
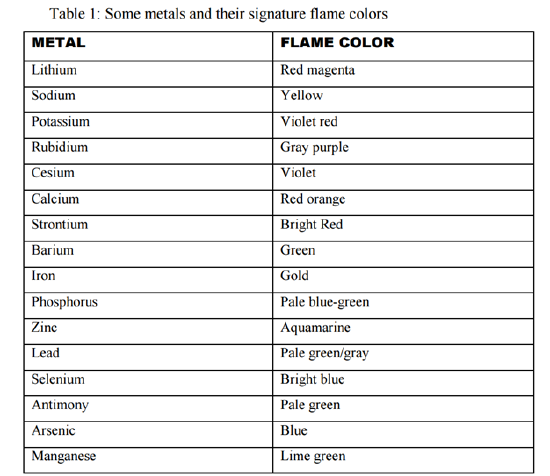
The flame test can be used to identify the following cations. Dip a clean wire loop into a solid sample of the compound being tested. Small cup of water. A metal salt consists of a component cation the metal and an anion. The flame test can be used to identify the following cations.
 Source: socratic.org
Source: socratic.org
Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Container of water to douse the flame. Small cup of water. Primarily the flame test detects the presence of metal ions in a compound and as ions of each element have a specific characteristic based in their emission spectrum the flame test for every element is different and distinctive. Small glass dish for each powder tested.
 Source: hightechhigh-faithsdp.weebly.com
Source: hightechhigh-faithsdp.weebly.com
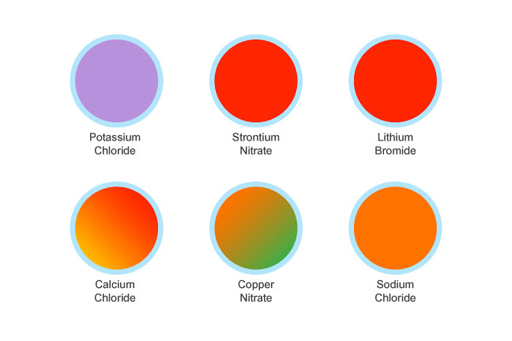
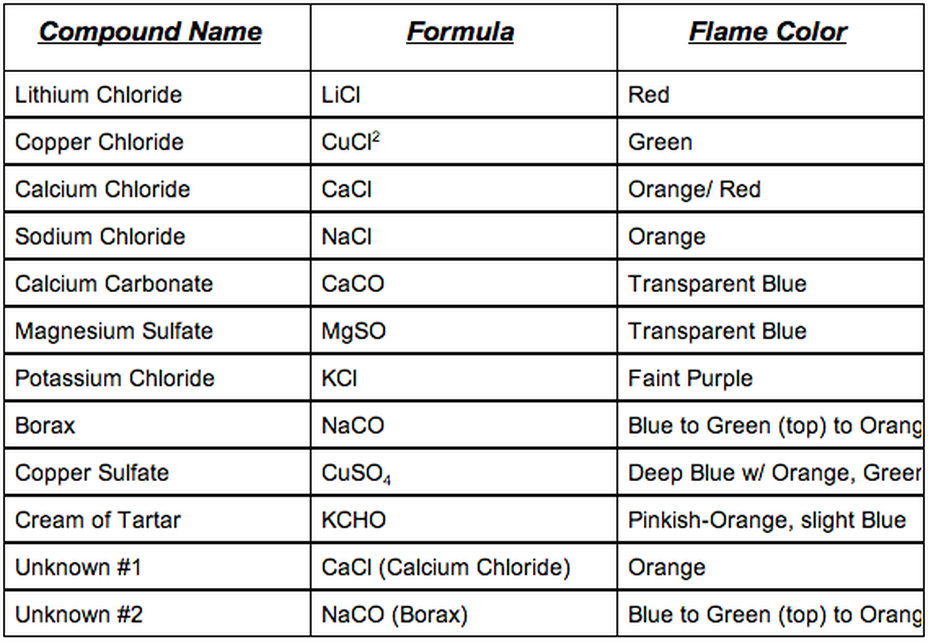
The flame test can be used to identify the following cations. The flame test can be used to distinguish between the oxidation states of atoms of a single element too. All of my results matched with the expected colors listed in the background. To carry out a flame test. The elements that had similar results in the flame test were lithium and strontium.

About press copyright contact us creators advertise developers terms privacy policy safety how youtube works. As well as copper and lead and barium and manganese. It is widely used to detect and analyze the presence of certain elements in the given salt or compound. Small cup of water. Li na k ca s.
 Source: compoundchem.com
Source: compoundchem.com
Put the loop into the edge of the blue flame from a bunsen burner. The anion can affect the result of the flame test. For group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. Here are a couple of household materials that contain metals that are easily seen and identifiable in a flame test. This is the basis of flame tests.
 Source: daviddarling.info
Source: daviddarling.info
All of my results matched with the expected colors listed in the background. As well as copper and lead and barium and manganese. Not all metal ions give flame colours. The flame test can be used to distinguish between the oxidation states of atoms of a single element too. Dip a clean wire loop into a solid sample of the compound being tested.
 Source: compoundchem.com
Source: compoundchem.com
However the range of elements positively detectable under these conditions is small as the test relies on the subjective experience of the experimenter rather than any objective measurements. This is the basis of flame tests. Colored flames from strontium cesium sodium and lithium from left to right. Carrying out a flame test. Li na k ca s.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title flame test elements by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







