How are mixtures and compounds alike
How Are Mixtures And Compounds Alike. They both are poliatomic. Compounds are pure substances. Compounds are atoms these atoms can be the same element or different. In a compound the ingredients are present in a definite proportion.
 Mixtures And Compounds Lab From mg-door.com
Mixtures And Compounds Lab From mg-door.com
Compound are substances which are formed by chemically combining two or more elements. Compounds are homogeneous they are pure substances. Mixtures are substances that are formed by physically mixing two or more substances. They are made from the same types of molecules. The constituents or components of a mixture and compound can easily be separated. Heterogeneous mixtures are those that are not uniform in composition or those that contain different substances.
Mixtures and compounds are alike in the manner that they are made up of two or more different parts.
Both compounds and mixtures have physical and chemical properties. Although chemists most often classify matter. Compounds are pure substances. For example soil is a combination of many different substances is non uniform in composition and thus a heterogeneous mixture. They are different in the manner that a compound usually consists of elements being chemically. Compounds are three types which are covalent compounds metallic compounds and ionic compounds.
Source: quora.com
Each molecule of a compound is made from two or more different kinds of atoms that are chemically bonded. However the way in which those elements are held together and the ratio of one element to the other significantly separates these two states of matter chemically. Mixtures and compounds are alike in the manner that they are made up of two or more different parts. The compound is a pure substance which contains only one kind of molecule. They both are poliatomic.
 Source: slideplayer.com
Source: slideplayer.com
Compounds are three types which are covalent compounds metallic compounds and ionic compounds. The compound is a pure substance which contains only one kind of molecule. A compound is a combination of multiple elements. Compounds are homogeneous they are pure substances. Compounds are atoms these atoms can be the same element or different.
 Source: socratic.org
Source: socratic.org
Heterogeneous mixtures are those that are not uniform in composition or those that contain different substances. On the contrary the constituents are present in a variable proportion in a mixture. Compounds are homogeneous they are pure substances. Compounds are three types which are covalent compounds metallic compounds and ionic compounds. Although chemists most often classify matter.
 Source: mg-door.com
Source: mg-door.com
Both compound and mixture consist of two or more substances elements. Compounds like mixtures can be separated by similar methods. Compounds are always homogeneous whereas mixtures can be homogeneous or heterogeneous. Suspensions colloids and alloys are other examples of this type. Both compound and mixture are combined in a definite ratio or in any proportion.
 Source: diffen.com
Source: diffen.com
Compounds and mixtures are alike because they both represent classifications of matter which contain more than one chemical element. Both compound and mixture are combined in a definite ratio or in any proportion. Compounds like mixtures can be separated by similar methods. Compounds are chemically bonded have formulas showing how many atoms of each type and the ratios they take a chemical change to separate. Compounds are always homogeneous whereas mixtures can be homogeneous or heterogeneous.
 Source: slideplayer.com
Source: slideplayer.com
In a compound the ingredients are present in a definite proportion. Heterogeneous mixtures are those that are not uniform in composition or those that contain different substances. The constituents or components of a mixture and compound can easily be separated. Mixtures are substances that are formed by physically mixing two or more substances. Although chemists most often classify matter.
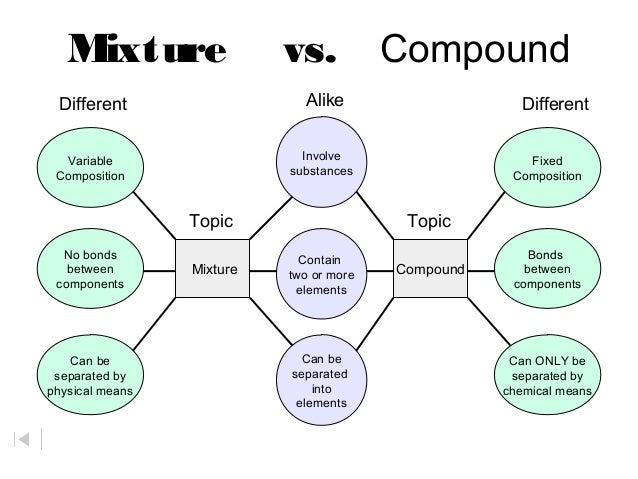 Source: slideshare.net
Source: slideshare.net
Compounds are atoms these atoms can be the same element or different. Both compounds and mixtures have physical and chemical properties. Each molecule of a compound is made from two or more different kinds of atoms that are chemically bonded. Heterogeneous mixtures are those that are not uniform in composition or those that contain different substances. In a compound the ingredients are present in a definite proportion.
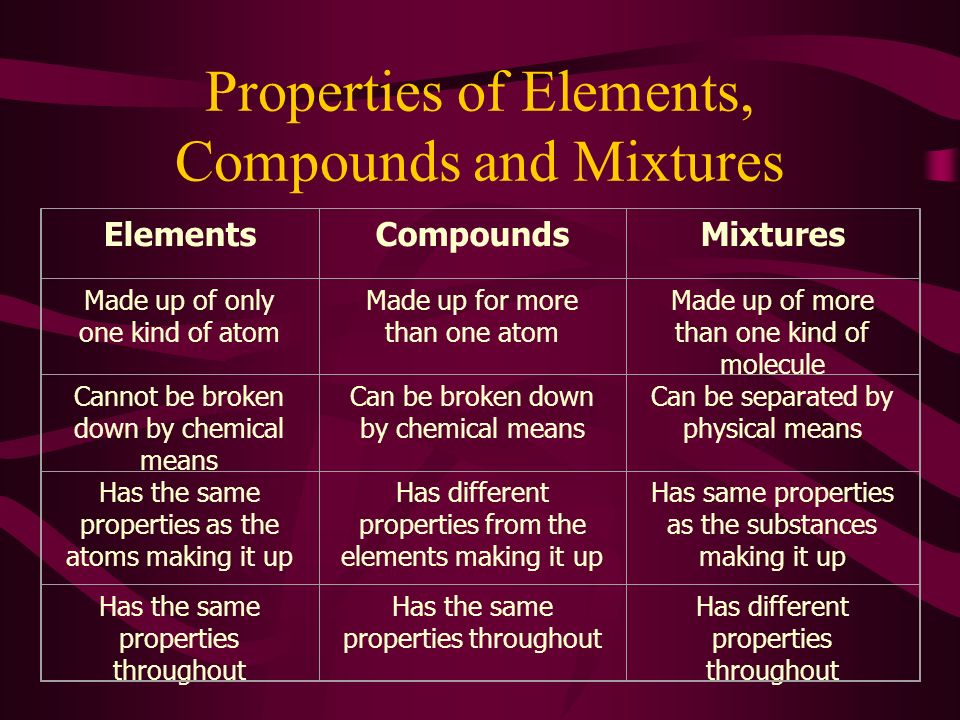 Source: slideplayer.com
Source: slideplayer.com
Compounds are homogeneous they are pure substances. Compounds are homogeneous they are pure substances. They both are poliatomic. Suspensions colloids and alloys are other examples of this type. Compounds are atoms these atoms can be the same element or different.
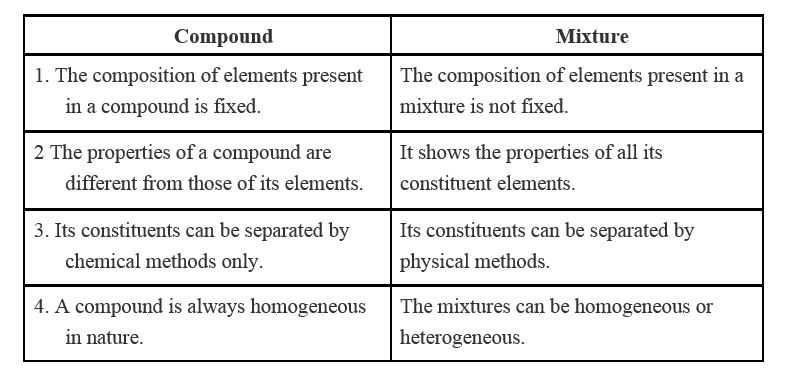
Although chemists most often classify matter. Mixtures are substances that are formed by physically mixing two or more substances. They do not contain any atomic bonds. Compounds are three types which are covalent compounds metallic compounds and ionic compounds. They are made from the same types of molecules.
 Source: slideplayer.com
Source: slideplayer.com
The compound is a pure substance which contains only one kind of molecule. Compounds are chemically bonded have formulas showing how many atoms of each type and the ratios they take a chemical change to separate. They do not contain any atomic bonds. H20 nh3 o2 cl2 s8 the easiest way to define a mixture is a combination of compounds. They both are poliatomic.
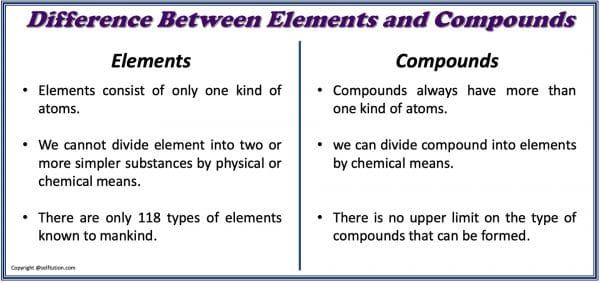 Source: selftution.com
Source: selftution.com
Compounds are three types which are covalent compounds metallic compounds and ionic compounds. Compounds are atoms these atoms can be the same element or different. Compounds are always homogeneous whereas mixtures can be homogeneous or heterogeneous. Mixtures are substances that are formed by physically mixing two or more substances. Both compound and mixture are combined in a definite ratio or in any proportion.
 Source: teachervision.com
Source: teachervision.com
They are different in the manner that a compound usually consists of elements being chemically. H20 nh3 o2 cl2 s8 the easiest way to define a mixture is a combination of compounds. On the contrary the constituents are present in a variable proportion in a mixture. The compound is a pure substance which contains only one kind of molecule. Mixtures and compounds are alike in the manner that they are made up of two or more different parts.
 Source: slideserve.com
Source: slideserve.com
Both compound and mixture are combined in a definite ratio or in any proportion. Compounds are atoms these atoms can be the same element or different. On the contrary the constituents are present in a variable proportion in a mixture. A compound is a combination of multiple elements. Compounds are chemically bonded have formulas showing how many atoms of each type and the ratios they take a chemical change to separate.
Source: quora.com
Compounds are pure substances. Both compound and mixture consist of two or more substances elements. The constituents or components of a mixture and compound can easily be separated. In a compound the ingredients are present in a definite proportion. They are made from the same types of molecules.
 Source: slideplayer.com
Source: slideplayer.com
On the contrary the constituents are present in a variable proportion in a mixture. Compounds are three types which are covalent compounds metallic compounds and ionic compounds. Compounds are atoms these atoms can be the same element or different. Compounds and mixtures are alike because they both represent classifications of matter which contain more than one chemical element. However the way in which those elements are held together and the ratio of one element to the other significantly separates these two states of matter chemically.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how are mixtures and compounds alike by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







