How are mixtures different from pure substances
How Are Mixtures Different From Pure Substances. Chemical physical properties. Gold silver iron and aluminium are pure substances to name a few. Pure substances are further divided into elements and compounds. As a result pure substances can t be separated into other materials but the different properties of the components of mixtures can be used to separate them into pure substances.
 Lesson Categories Of Chemicals And Mixtures From serpmedia.org
Lesson Categories Of Chemicals And Mixtures From serpmedia.org
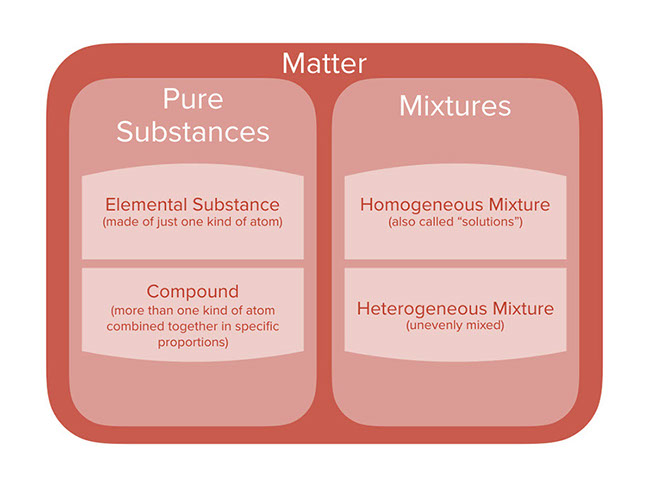
Pure substances are further divided into elements and compounds. It can be the same molecule or atom. A pure substance is often described as substances that are made up of only one type of atom or molecule. However matter can also be classified into pure substances and mixtures. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. By their chemical composition pure substances get divided into two types elements and compounds.
However matter can also be classified into pure substances and mixtures.
However matter can also be classified into pure substances and mixtures. By their chemical composition pure substances get divided into two types elements and compounds. Pure substances are made up of one kind of material with consistent properties while mixtures consist of two or more pure substances each having different properties. What is a mixture. A pure substance contains only one kind of compound. Mixture is a combination of two or more pure substances where each substance keeps its own identity upon mixing.
 Source: socratic.org
Source: socratic.org
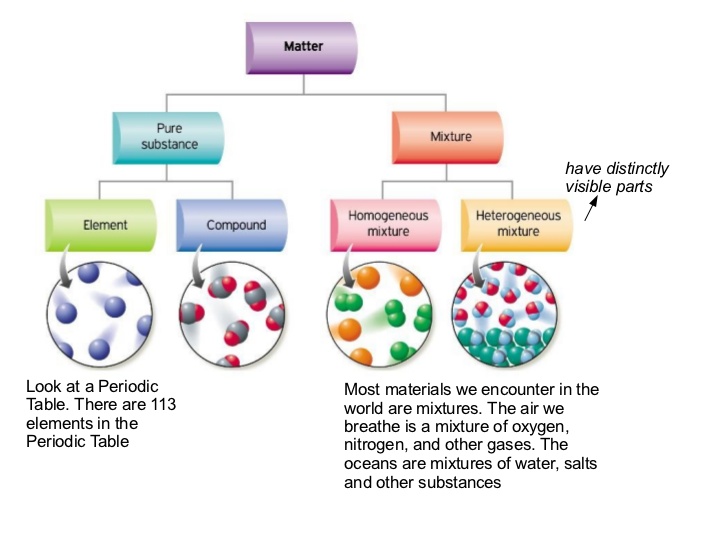
Substances which have a specific composition and cannot be separated into any constituents are called pure substances. The combination of two or more pure substances is called a mixture. Pure substances are further divided into elements and compounds. The basic difference between pure substance and mixture is that pure substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components retains its characteristic properties. Examples of compounds include water glucose salt and carbon dioxide.
 Source: sciencesfp.com
Source: sciencesfp.com
Substances which have a specific composition and cannot be separated into any constituents are called pure substances. As a result pure substances can t be separated into other materials but the different properties of the components of mixtures can be used to separate them into pure substances. Pure substances are made up of one kind of material with consistent properties while mixtures consist of two or more pure substances each having different properties. Chemical physical properties. However matter can also be classified into pure substances and mixtures.
Source: quora.com
By their chemical composition pure substances get divided into two types elements and compounds. 1 pure substance the substances that contain only one type of particle and they are free from any mixture are known as pure substances. Heterogeneous and homogeneous mixtures. It can be the same molecule or atom. By their chemical composition pure substances get divided into two types elements and compounds.
 Source: prezi.com
Source: prezi.com
Chemical physical properties. The combination of two or more pure substances is called a mixture. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. The known elements listed in the periodic table can be considered pure substances. Heterogeneous and homogeneous mixtures.
 Source: youtube.com
Source: youtube.com
Chemical physical properties. By their chemical composition pure substances get divided into two types elements and compounds. Heterogeneous and homogeneous mixtures. A few differences between pure substances and mixtures have been tabulated below. Examples of elements include hydrogen oxygen gold silver compounds are made up of different types of atoms joined together by chemical bonds.
 Source: aplustopper.com
Source: aplustopper.com
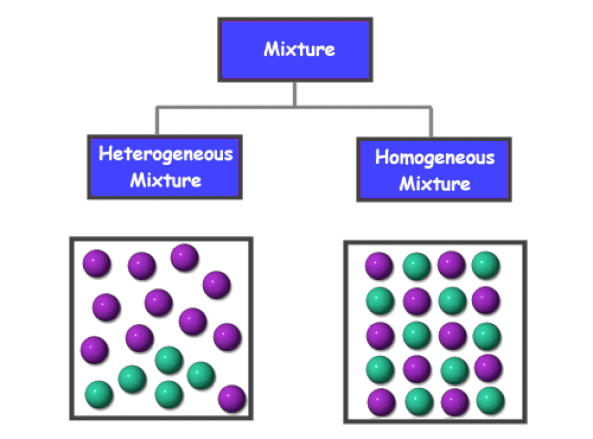
Mixtures can be classified into two types viz. Mixtures can be classified into two types viz. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Heterogeneous and homogeneous mixtures.
 Source: toppr.com
Source: toppr.com
It can be the same molecule or atom. Pure substances are made up of one kind of material with consistent properties while mixtures consist of two or more pure substances each having different properties. By their chemical composition pure substances get divided into two types elements and compounds. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. It can be the same molecule or atom.
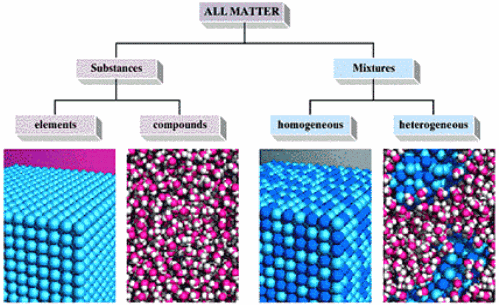 Source: majordifferences.com
Source: majordifferences.com
A pure substance contains only one kind of compound. Mixtures are composed of several kinds of compounds. Mixture is a combination of two or more pure substances where each substance keeps its own identity upon mixing. By their chemical composition pure substances get divided into two types elements and compounds. Substances which have a specific composition and cannot be separated into any constituents are called pure substances.
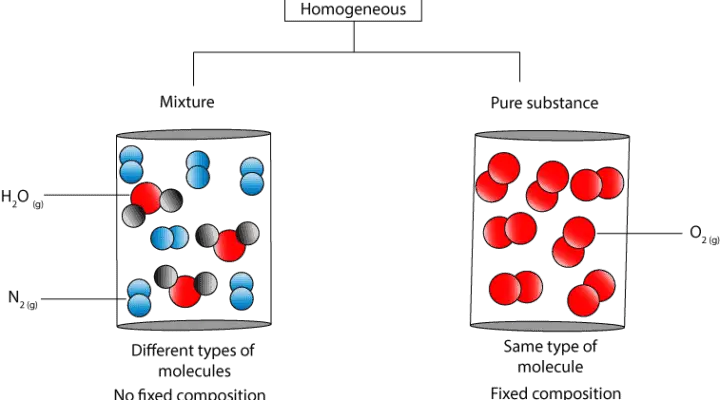 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Heterogeneous and homogeneous mixtures. It can be the same molecule or atom. By their chemical composition pure substances get divided into two types elements and compounds. Mixtures can be classified into two types viz. However matter can also be classified into pure substances and mixtures.
 Source: sciencenotes.org
Source: sciencenotes.org
By their chemical composition pure substances get divided into two types elements and compounds. Mixtures are composed of several kinds of compounds. Chemical physical properties. A pure substance is often described as substances that are made up of only one type of atom or molecule. Substances which have a specific composition and cannot be separated into any constituents are called pure substances.
 Source: pinterest.com
Source: pinterest.com
What is a mixture. Mixtures are composed of several kinds of compounds. The combination of two or more pure substances is called a mixture. By their chemical composition pure substances get divided into two types elements and compounds. Mixtures can be classified into two types viz.
 Source: bioprofe.com
Source: bioprofe.com
Gold silver iron and aluminium are pure substances to name a few. A pure substance is often described as substances that are made up of only one type of atom or molecule. Examples of compounds include water glucose salt and carbon dioxide. It can be the same molecule or atom. Mixtures are composed of several kinds of compounds.
 Source: serpmedia.org
Source: serpmedia.org
Mixtures are composed of several kinds of compounds. The combination of two or more pure substances is called a mixture. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. A pure substance contains only one kind of compound. As a result pure substances can t be separated into other materials but the different properties of the components of mixtures can be used to separate them into pure substances.
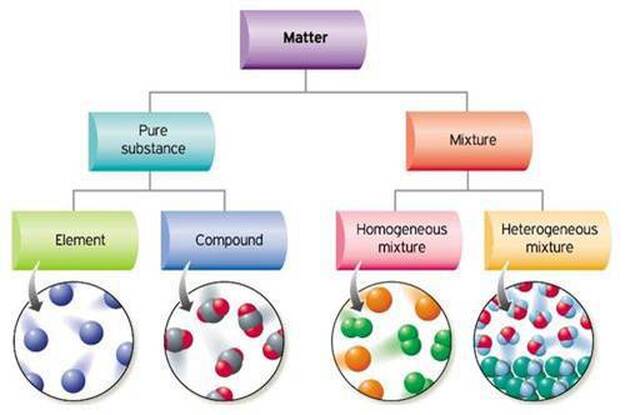 Source: sciencesfp.com
Source: sciencesfp.com
Examples of compounds include water glucose salt and carbon dioxide. A pure substance is often described as substances that are made up of only one type of atom or molecule. Mixture is a combination of two or more pure substances where each substance keeps its own identity upon mixing. The combination of two or more pure substances is called a mixture. As a result pure substances can t be separated into other materials but the different properties of the components of mixtures can be used to separate them into pure substances.
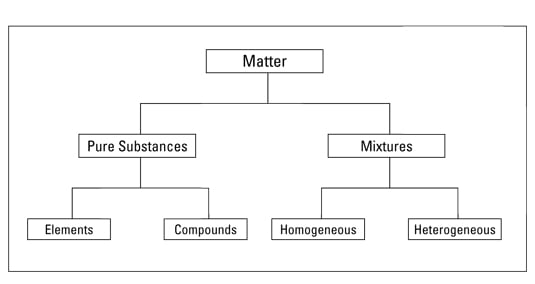 Source: dummies.com
Source: dummies.com
The main difference between pure substance and mixture lies in their composition. Mixtures are composed of several kinds of compounds. Mixture is a combination of two or more pure substances where each substance keeps its own identity upon mixing. The known elements listed in the periodic table can be considered pure substances. A pure substance contains only one kind of compound.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how are mixtures different from pure substances by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.