How to do copper plating
How To Do Copper Plating. Now place the copper into the warm vinegar peroxide mixture. This is probably the easiest way to create reliable attractive copper plate on many different metals. Electrolyze this solution for 10 minutes or so. Make sure to not polish to much sins it is only a thin layer of copper.
 Plating With Copper Copper Plating Services Copper Electroplating Applications From sharrettsplating.com
Plating With Copper Copper Plating Services Copper Electroplating Applications From sharrettsplating.com
To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating. This is probably the easiest way to create reliable attractive copper plate on many different metals. Plating can be tricky but this method comes as close. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this.
Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object.
Electrolyze this solution for 10 minutes or so. Make sure to not polish to much sins it is only a thin layer of copper. Screw on the lid and gently swirl the liquid and copper in the jar. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object.
 Source: m.youtube.com
Source: m.youtube.com
The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. Make sure to not polish to much sins it is only a thin layer of copper. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. Electrolyze this solution for 10 minutes or so.
 Source: metallurgyfordummies.com
Source: metallurgyfordummies.com
To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. This is probably the easiest way to create reliable attractive copper plate on many different metals. Make sure to not polish to much sins it is only a thin layer of copper. Electrolyze this solution for 10 minutes or so. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper.
 Source: wikiwand.com
Source: wikiwand.com
The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. This is probably the easiest way to create reliable attractive copper plate on many different metals. Screw on the lid and gently swirl the liquid and copper in the jar. Electrolyze this solution for 10 minutes or so. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper.
 Source: researchgate.net
Source: researchgate.net
Electrolyze this solution for 10 minutes or so. This is probably the easiest way to create reliable attractive copper plate on many different metals. Electrolyze this solution for 10 minutes or so. As time passes the mixture will become more and more blue. Now place the copper into the warm vinegar peroxide mixture.
 Source: pfonline.com
Source: pfonline.com
Electrolyze this solution for 10 minutes or so. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. Screw on the lid and gently swirl the liquid and copper in the jar. Now place the copper into the warm vinegar peroxide mixture. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this.
 Source: dupont.com
Source: dupont.com
Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. As time passes the mixture will become more and more blue. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. Plating can be tricky but this method comes as close. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating.
 Source: youtube.com
Source: youtube.com
As time passes the mixture will become more and more blue. Electrolyze this solution for 10 minutes or so. This is probably the easiest way to create reliable attractive copper plate on many different metals. Screw on the lid and gently swirl the liquid and copper in the jar. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin.
 Source: instructables.com
Source: instructables.com
Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. Plating can be tricky but this method comes as close. This is probably the easiest way to create reliable attractive copper plate on many different metals. Screw on the lid and gently swirl the liquid and copper in the jar.
 Source: advancedplatingtech.com
Source: advancedplatingtech.com
Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. Make sure to not polish to much sins it is only a thin layer of copper. Electrolyze this solution for 10 minutes or so.
 Source: m.youtube.com
Source: m.youtube.com
To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. Plating can be tricky but this method comes as close. Electrolyze this solution for 10 minutes or so. Make sure to not polish to much sins it is only a thin layer of copper. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper.
 Source: chemedx.org
Source: chemedx.org
As time passes the mixture will become more and more blue. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. Electrolyze this solution for 10 minutes or so. This is probably the easiest way to create reliable attractive copper plate on many different metals. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin.
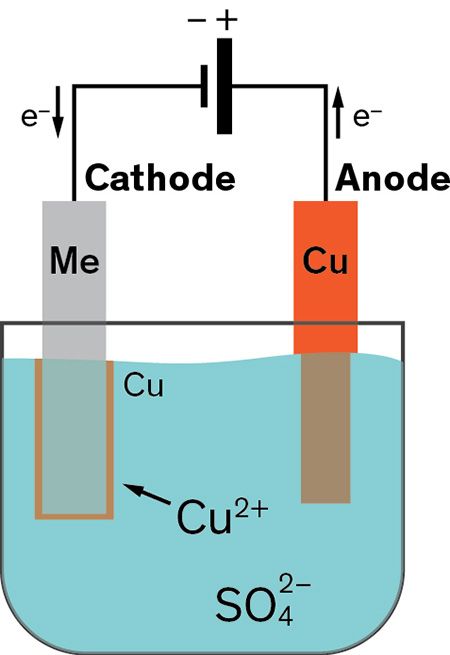
Electrolyze this solution for 10 minutes or so. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating. Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. Make sure to not polish to much sins it is only a thin layer of copper.
 Source: sharrettsplating.com
Source: sharrettsplating.com
Screw on the lid and gently swirl the liquid and copper in the jar. Make sure to not polish to much sins it is only a thin layer of copper. Now place the copper into the warm vinegar peroxide mixture. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin.
 Source: m.youtube.com
Source: m.youtube.com
Screw on the lid and gently swirl the liquid and copper in the jar. Make sure to not polish to much sins it is only a thin layer of copper. To get the plating shiny you can simply polish your object then its required to have a polisher and polish for copper. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating.
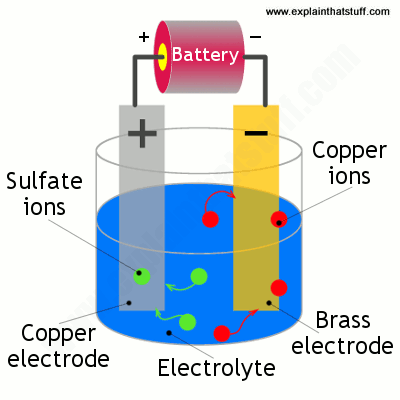 Source: explainthatstuff.com
Source: explainthatstuff.com
Do not forget to play around with the time and voltage to see the different results and try to get an even spread of copper over your object. Electrolyze this solution for 10 minutes or so. Screw on the lid and gently swirl the liquid and copper in the jar. Now place the copper into the warm vinegar peroxide mixture. Make sure to not polish to much sins it is only a thin layer of copper.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to do copper plating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.