Ice cube with salt
Ice Cube With Salt. The ice will cool the water down and the salt will allow the water temperature to drop below 0 c. The ice cube without salt melts because the air around it is warmer than 32 degrees f. The water cooled down further and re froze around the string. To see how nacl table salt melts ice by lowers the melting temperature of water you ll need an ice cube a glass of water and a piece of kitchen twine or string about 6 inches long and salt.
 Ice Cube On A String Experiment Little Passports From littlepassports.com
Ice Cube On A String Experiment Little Passports From littlepassports.com
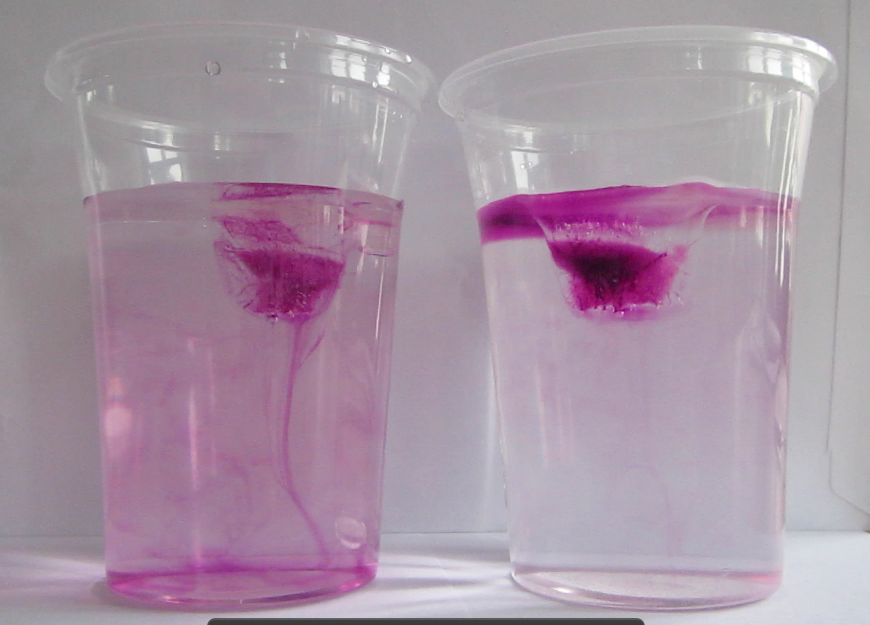
I had a question. In the left cup the cold meltwater from the ice cube is denser than the lukewarm water in the cup. Place the sealed smaller bag inside the gallon sized bag. The salted cube melts faster. When you make ice cubes colder by putting salt on them you have to be careful not to hold them in your hands because they can give your skin burns. Dyed ice cubes about to be put into fresh water left and salt water right when the ice cubes start melting it becomes very clear that they do so in different manners.
I figured that i may as well share the answer my blog.
I had a question. To see how nacl table salt melts ice by lowers the melting temperature of water you ll need an ice cube a glass of water and a piece of kitchen twine or string about 6 inches long and salt. Place the sealed smaller bag inside the gallon sized bag. The beer will then be fully submersed in sub zero water maximizing the surface area in contact. I figured that i may as well share the answer my blog. Try to pick the ice cube up without your fingers by simply placing the string on it and pulling up.
 Source: youtube.com
Source: youtube.com
How can this be. Have you ever wondered why putting chemicals like salt on a road makes the ice melt. The ice will cool the water down and the salt will allow the water temperature to drop below 0 c. The salt lowers the melting point of ice and stops roads from becoming icy and dangerous. Seal the larger ziploc bag shut.
 Source: twinkl.co.za
Source: twinkl.co.za
The water cooled down further and re froze around the string. I had a question. Put ice cubes into the gallon sized bag and add the salt. When you added salt to the ice cube the salt lowered the melting point of the ice. To see how nacl table salt melts ice by lowers the melting temperature of water you ll need an ice cube a glass of water and a piece of kitchen twine or string about 6 inches long and salt.
Source: alcademics.com
The ice cube without salt melts because the air around it is warmer than 32 degrees f. When you added salt to the ice cube the salt lowered the melting point of the ice. The water cooled down further and re froze around the string. When you add salt it dissolves into the water of the ice cube. Place the sealed smaller bag inside the gallon sized bag.
 Source: sciencebuddies.org
Source: sciencebuddies.org
The salt lowers the melting point of ice and stops roads from becoming icy and dangerous. When you add salt it dissolves into the water of the ice cube. Try to pick the ice cube up without your fingers by simply placing the string on it and pulling up. The water cooled down further and re froze around the string. We use salt in winter to prevent roads from getting icy.
 Source: greensborosciencecenter.wordpress.com
Source: greensborosciencecenter.wordpress.com
Dyed ice cubes about to be put into fresh water left and salt water right when the ice cubes start melting it becomes very clear that they do so in different manners. Place the sealed smaller bag inside the gallon sized bag. How can this be. Without salt the water will remain at a temperature slightly higher than 0 c even though you have 18 c ice cubes floating in it. Salt water freezes at a lower temperature than the 32 degrees f at which freshwater freezes.
 Source: mirjamglessmer.com
Source: mirjamglessmer.com
The truth is salt doesn t actually lower the temperature of the ice cubes it just lowers their freezing point which is the same as saying it lowers their melting point. I figured that i may as well share the answer my blog. Have you ever wondered why putting chemicals like salt on a road makes the ice melt. Salt is used to help melt ice and prevent it from re freezing on roads and walkways yet if you compare the melting of ice cubes in fresh water and salt water you ll find ice actually melts more slowly in the saline and the temperature gets colder. Dyed ice cubes about to be put into fresh water left and salt water right when the ice cubes start melting it becomes very clear that they do so in different manners.
 Source: onlypassionatecuriosity.com
Source: onlypassionatecuriosity.com
In the left cup the cold meltwater from the ice cube is denser than the lukewarm water in the cup. Place the sealed smaller bag inside the gallon sized bag. When you added salt to the ice cube the salt lowered the melting point of the ice. Salt is used to help melt ice and prevent it from re freezing on roads and walkways yet if you compare the melting of ice cubes in fresh water and salt water you ll find ice actually melts more slowly in the saline and the temperature gets colder. Drop an ice cube in a glass of ice water.
 Source: science-sparks.com
Source: science-sparks.com
The water cooled down further and re froze around the string. I figured that i may as well share the answer my blog. Salt water freezes at a lower temperature than the 32 degrees f at which freshwater freezes. The ice will cool the water down and the salt will allow the water temperature to drop below 0 c. I had a question.
 Source: ladbible.com
Source: ladbible.com
When you make ice cubes colder by putting salt on them you have to be careful not to hold them in your hands because they can give your skin burns. The truth is salt doesn t actually lower the temperature of the ice cubes it just lowers their freezing point which is the same as saying it lowers their melting point. The ice will cool the water down and the salt will allow the water temperature to drop below 0 c. Some interesting science occurs when you mix salt and ice. The salted cube melts faster.
 Source: sheknows.com
Source: sheknows.com
The beer will then be fully submersed in sub zero water maximizing the surface area in contact. I figured that i may as well share the answer my blog. Some interesting science occurs when you mix salt and ice. The water cooled down further and re froze around the string. The salt lowers the melting point of ice and stops roads from becoming icy and dangerous.
 Source: sciensation.org
Source: sciensation.org
I had a question. Dyed ice cubes about to be put into fresh water left and salt water right when the ice cubes start melting it becomes very clear that they do so in different manners. Salt is used to help melt ice and prevent it from re freezing on roads and walkways yet if you compare the melting of ice cubes in fresh water and salt water you ll find ice actually melts more slowly in the saline and the temperature gets colder. Some interesting science occurs when you mix salt and ice. The ice will cool the water down and the salt will allow the water temperature to drop below 0 c.
 Source: sciensation.org
Source: sciensation.org
How can this be. Some interesting science occurs when you mix salt and ice. In the left cup the cold meltwater from the ice cube is denser than the lukewarm water in the cup. Put ice cubes into the gallon sized bag and add the salt. Drop an ice cube in a glass of ice water.
 Source: littlepassports.com
Source: littlepassports.com
Salt is used to help melt ice and prevent it from re freezing on roads and walkways yet if you compare the melting of ice cubes in fresh water and salt water you ll find ice actually melts more slowly in the saline and the temperature gets colder. The water cooled down further and re froze around the string. When you add salt it dissolves into the water of the ice cube. Place the sealed smaller bag inside the gallon sized bag. We use salt in winter to prevent roads from getting icy.
 Source: thoughtco.com
Source: thoughtco.com
In the left cup the cold meltwater from the ice cube is denser than the lukewarm water in the cup. Put ice cubes into the gallon sized bag and add the salt. Salt water freezes at a lower temperature than the 32 degrees f at which freshwater freezes. When you add salt it dissolves into the water of the ice cube. Salt is used to help melt ice and prevent it from re freezing on roads and walkways yet if you compare the melting of ice cubes in fresh water and salt water you ll find ice actually melts more slowly in the saline and the temperature gets colder.
![]() Source: knowyourmeme.com
Source: knowyourmeme.com
The beer will then be fully submersed in sub zero water maximizing the surface area in contact. When you added salt to the ice cube the salt lowered the melting point of the ice. When you add salt it dissolves into the water of the ice cube. In the left cup the cold meltwater from the ice cube is denser than the lukewarm water in the cup. I had a question.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ice cube with salt by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.