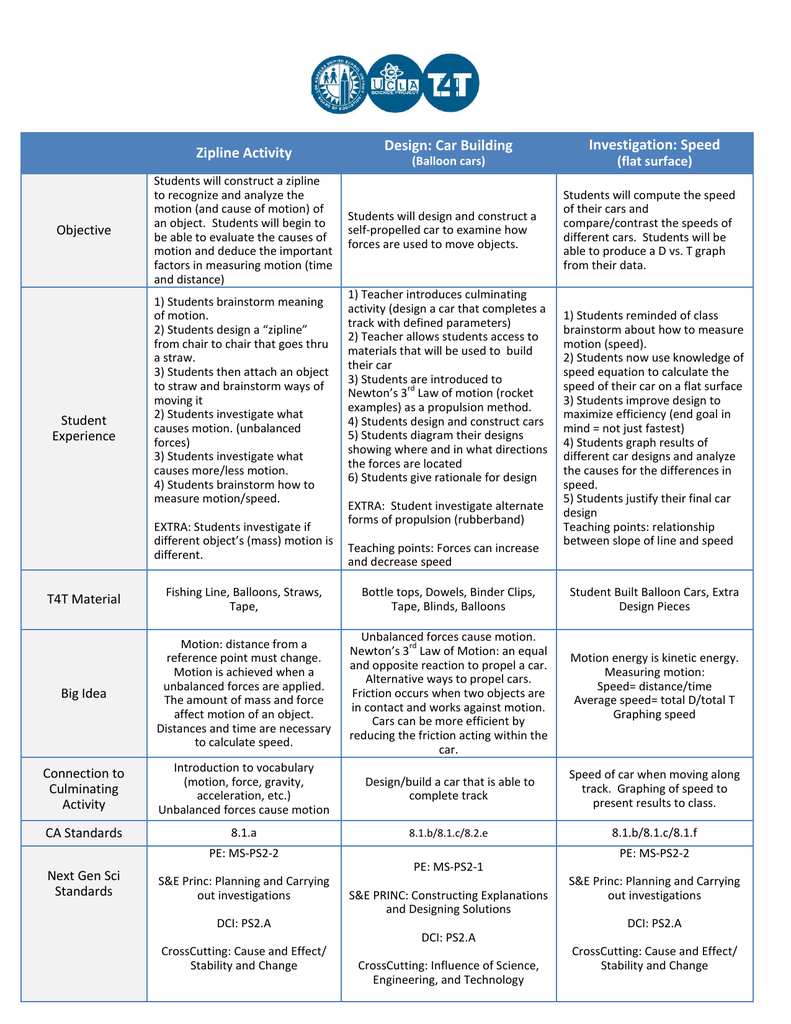
In the periodic table the most reactive metals are found
In The Periodic Table The Most Reactive Metals Are Found. In groups 13 through 16 in the center. The most reactive metal on the periodic table is francium. What did one chemist say to another when he found him aggrieved. Properties of periodic table.
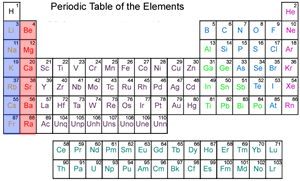 Chemistry Reactive Metals From dynamicscience.com.au
Chemistry Reactive Metals From dynamicscience.com.au
What did one chemist say to another when he found him aggrieved. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table. Periodic table view presentation slides online. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. In groups 13 through 16 in the center. In first column the alkali metals are particularly reactive.
The most reactive metal on the periodic table is francium.
Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. In periods 6 and 7 at the bottom. The most reactive metal on the periodic table is francium. In the periodic table the most reactive metals are found. Fluorine is identified as the most reactive nonmetal and the most electronegative element in the periodic table making it the strongest oxidizing agent.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
You might wonder how the alkali metals were ever discovered in nature if they react so violently to air and water. The most reactive metal in the periodic table is francium. In periods 6 and 7 at the bottom. The metals in that row in their purest forms are very very reactive. In the periodic table the most reactive metals are found.
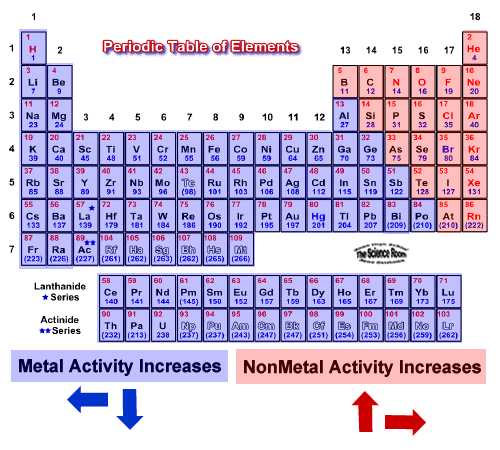 Source: socratic.org
Source: socratic.org
Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. In periods 6 and 7 at the bottom. These are the elements in the far left column with. The most reactive metal on the periodic table is francium. What did one chemist say to another when he found him aggrieved.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
The most reactive metal in the periodic table is francium. The most reactive metal in the periodic table is francium. The metals in that row in their purest forms are very very reactive. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive.
Source: quora.com
These metals are highly reactive because they all have only one valence electron. These metals are highly reactive because they all have only one valence electron. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. Well as it turns out most of the alkali metals are found in nature as ions. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive.
 Source: pinterest.com
Source: pinterest.com
In periods 6 and 7 at the bottom. These metals are highly reactive because they all have only one valence electron. You might wonder how the alkali metals were ever discovered in nature if they react so violently to air and water. In first column the alkali metals are particularly reactive. The elements that have the least amount of electrons in their valence shells tend to be the most reactive.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
The most reactive metals on the periodic table are the alkali metals which are found in group 1a. In period 1 the first row across the top. The elements that have the least amount of electrons in their valence shells tend to be the most reactive. Fluorine is identified as the most reactive nonmetal and the most electronegative element in the periodic table making it the strongest oxidizing agent. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table.
 Source: periodictable.me
Source: periodictable.me
In first column the alkali metals are particularly reactive. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table. These metals are highly reactive because they all have only one valence electron. What did one chemist say to another when he found him aggrieved. In period 1 the first row across the top.
 Source: modelscience.com
Source: modelscience.com
Well as it turns out most of the alkali metals are found in nature as ions. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. In group 1 the first column on the left. In first column the alkali metals are particularly reactive. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table.
 Source: toppr.com
Source: toppr.com
K the k potassium only needs to lose one electron to have a full valence radium needs to lose 2. Properties of periodic table. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. These are the elements in the far left column with. Elements in group 1 are more reactive than elements in group 2.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
These metals are highly reactive because they all have only one valence electron. In period 1 the first row across the top. The elements that have the least amount of electrons in their valence shells tend to be the most reactive. You might wonder how the alkali metals were ever discovered in nature if they react so violently to air and water. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions.
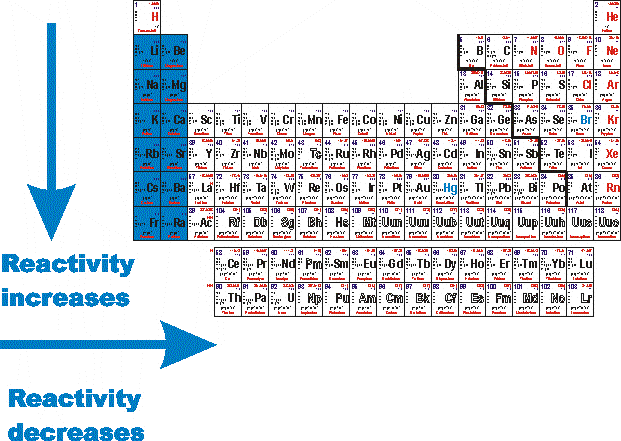 Source: socratic.org
Source: socratic.org
Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. The elements that have the least amount of electrons in their valence shells tend to be the most reactive. Properties of periodic table. In the periodic table the most reactive metals are found. Fluorine is identified as the most reactive nonmetal and the most electronegative element in the periodic table making it the strongest oxidizing agent.
 Source: interactivesciencenotebook.weebly.com
Source: interactivesciencenotebook.weebly.com
These metals are highly reactive because they all have only one valence electron. In period 1 the first row across the top. Properties of periodic table. What did one chemist say to another when he found him aggrieved. The metals in that row in their purest forms are very very reactive.
 Source: slideplayer.com
Source: slideplayer.com
These are the elements in the far left column with. What did one chemist say to another when he found him aggrieved. The most reactive metals on the periodic table are the alkali metals which are found in group 1a. In periods 6 and 7 at the bottom. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table.
Source: quora.com
In first column the alkali metals are particularly reactive. These are the elements in the far left column with. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. Properties of periodic table.
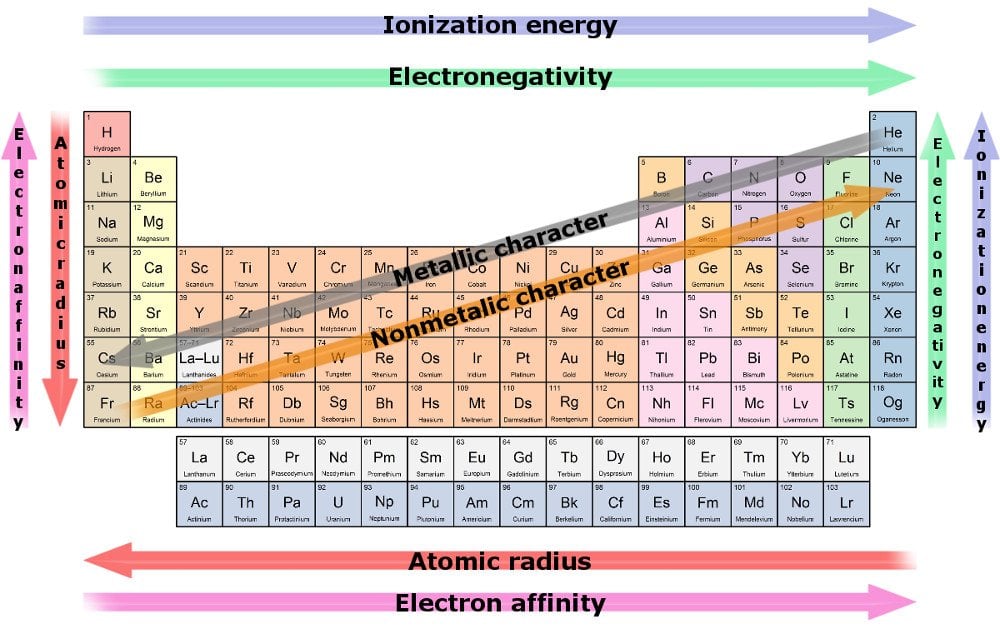 Source: scienceabc.com
Source: scienceabc.com
You might wonder how the alkali metals were ever discovered in nature if they react so violently to air and water. The elements also increase in atomic radius decrease in electronegativity and decrease in melting and boiling points as you move down the periodic table. In groups 13 through 16 in the center. These are the elements in the far left column with. In group 1 the first column on the left.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title in the periodic table the most reactive metals are found by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.