Indicator color change acid or base
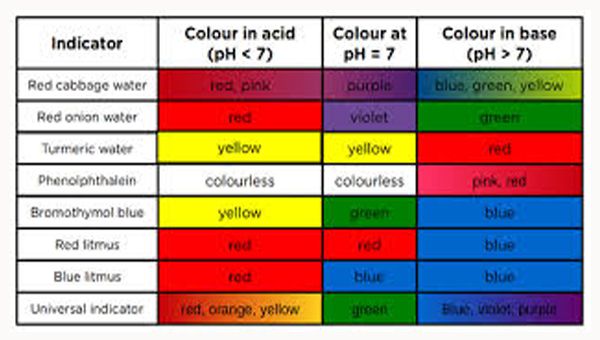
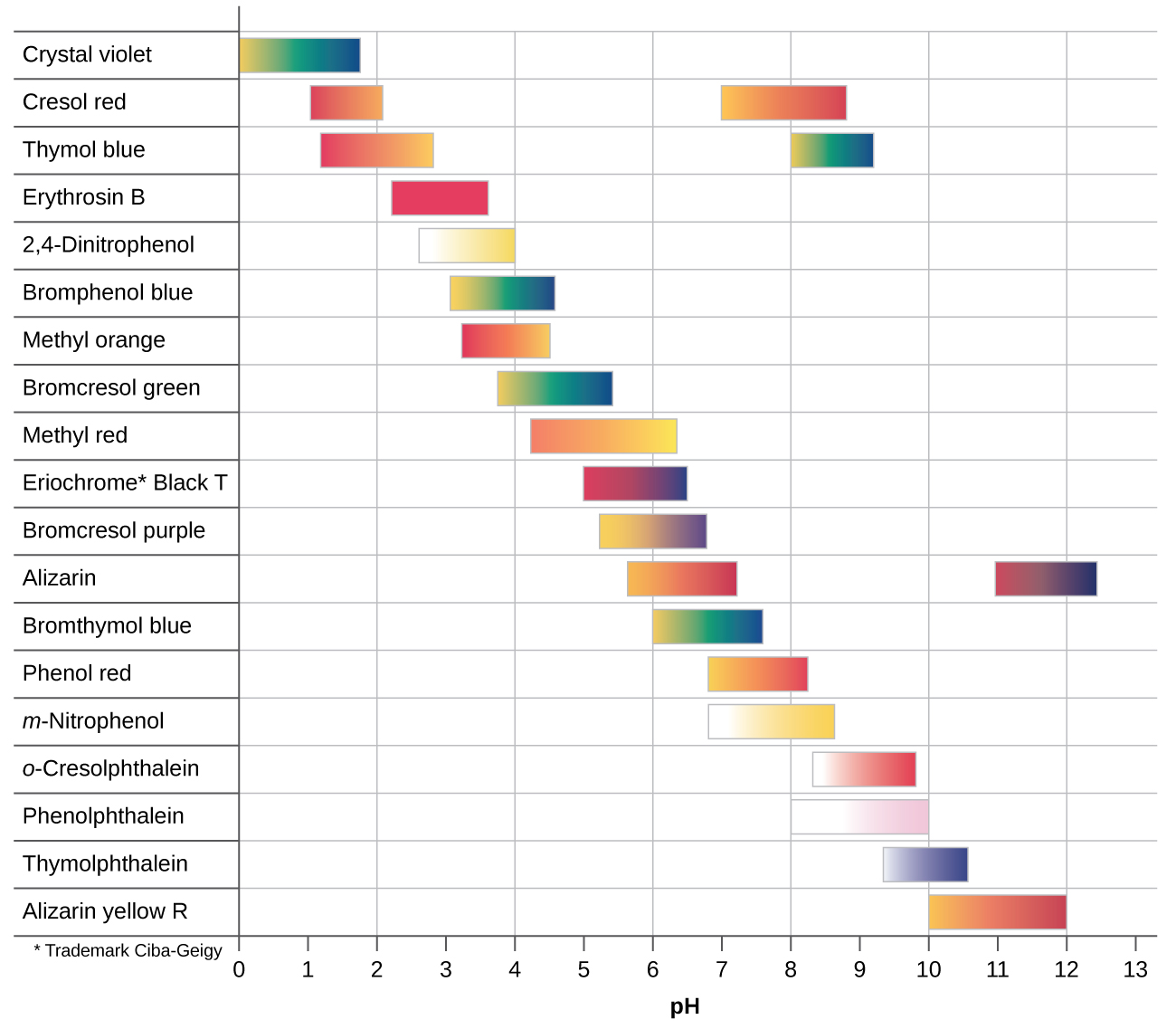
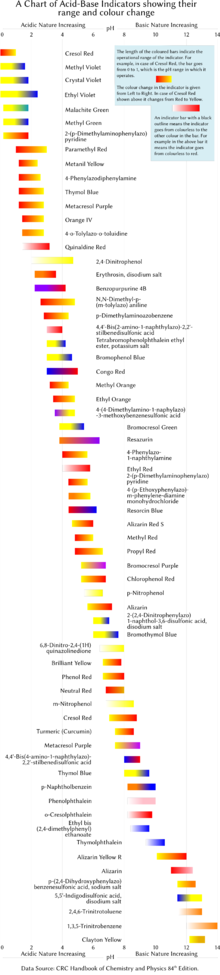
Indicator Color Change Acid Or Base. These indicators are used to identify the termination of an acid base reaction. An acid base indicator is either a weak acid or weak base that exhibits a color change as the concentration of hydrogen h or hydroxide oh ions changes in an aqueous solution. Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. The ranges for the color changes are given in the table below the figure together with the corresponding pk a value of the indicators.
 Types Of Indicators In Chemistry With Examples Applications From guidancecorner.com
Types Of Indicators In Chemistry With Examples Applications From guidancecorner.com
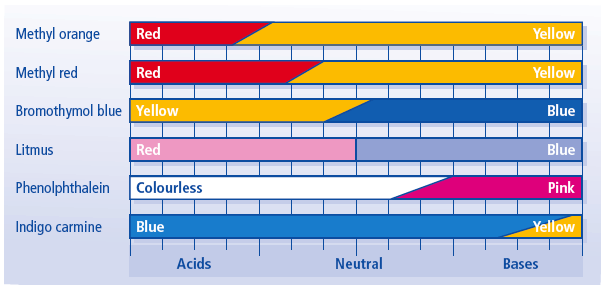
Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. The molecule structure of each indicator is shown below the table. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. Acid base indicators are chemical substances that can give a color change in a reaction medium as a response to a change in ph. They are also called ph indicators. The most widely known indicator is litmus paper which turns red in acidic solutions and blue in basic solutions.
Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction.
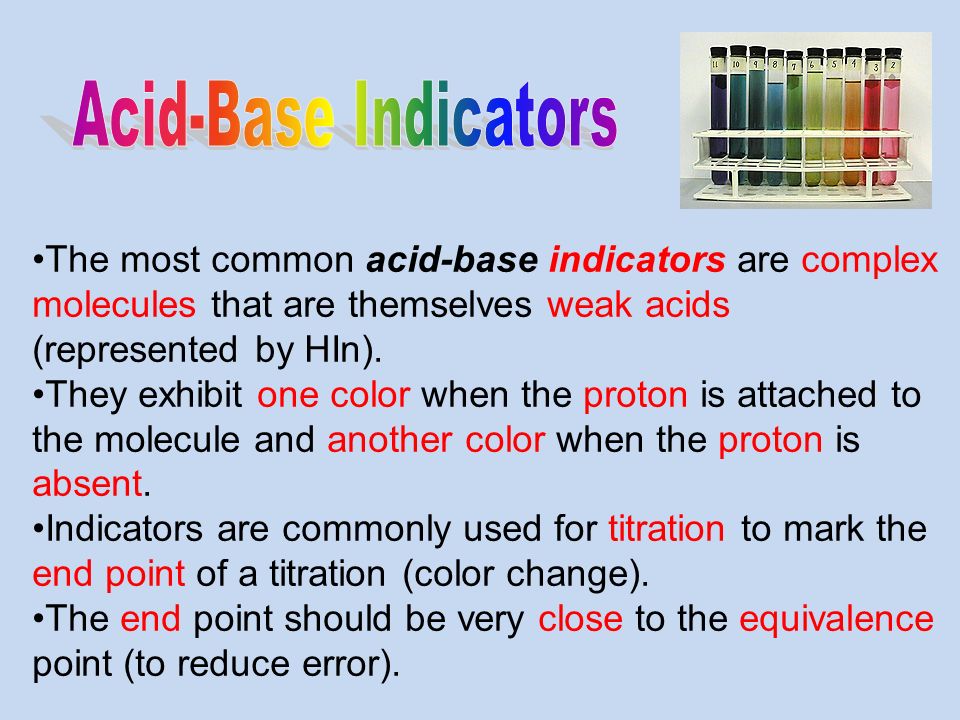
Most of the indicators are themselves weak acids. An indicator is a large organic molecule that works somewhat like a color dye. However it is easy to make your own. Most of the indicators are themselves weak acids. So how do scientists know if a substance is an acid or a base. The undissociated form of the indicator is a different color than the iogenic form of the indicator.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
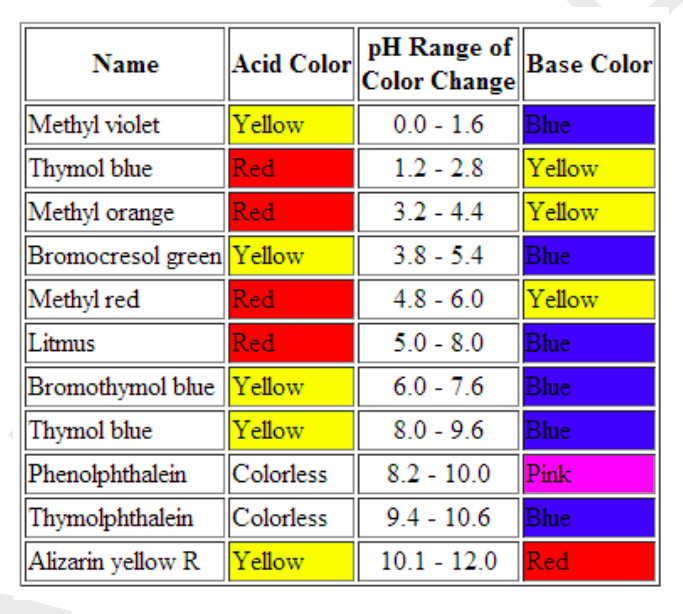
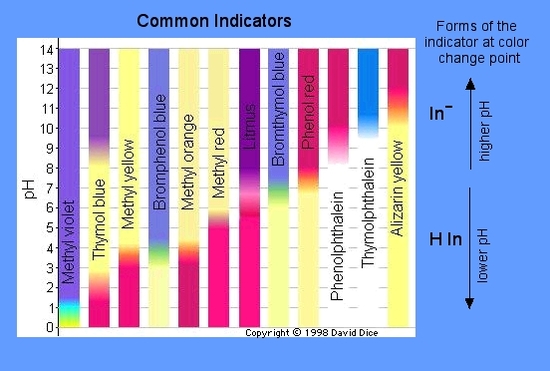
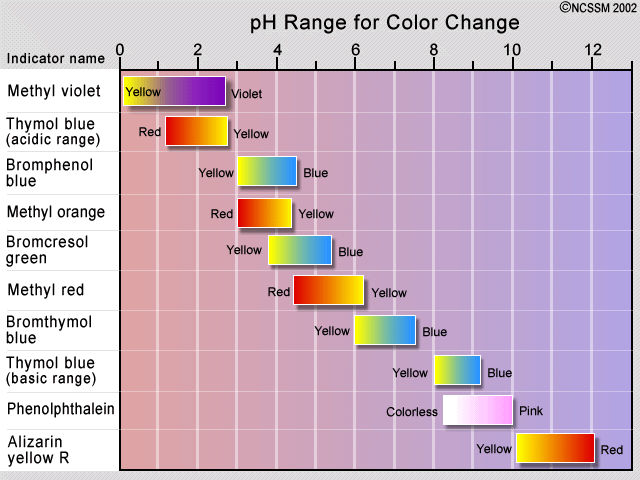
An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with. An indicator is a large organic molecule that works somewhat like a color dye. Acid base indicators are chemical substances that can give a color change in a reaction medium as a response to a change in ph. The ranges for the color changes are given in the table below the figure together with the corresponding pk a value of the indicators.
 Source: chem.libretexts.org
Source: chem.libretexts.org
An indicator is a large organic molecule that works somewhat like a color dye. An indicator is a dye that changes colour when it is put into an acid or a base. Most of the indicators are themselves weak acids. So how do scientists know if a substance is an acid or a base. They are also called ph indicators.
 Source: en.wikipedia.org
Source: en.wikipedia.org
They are also called ph indicators. One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with. The most widely known indicator is litmus paper which turns red in acidic solutions and blue in basic solutions. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations.
 Source: slideplayer.com
Source: slideplayer.com
An indicator is a large organic molecule that works somewhat like a color dye. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. Whereas most dyes do not change color with the amount of acid or base present there are many molecules known as acid base indicators which do respond to a change in the hydrogen ion concentration. These indicators are used to identify the termination of an acid base reaction. An acid base indicator is a weak acid or a weak base.
 Source: foundoutaboutchemistry.blogspot.com
Source: foundoutaboutchemistry.blogspot.com
These indicators are used to identify the termination of an acid base reaction. The ranges for the color changes are given in the table below the figure together with the corresponding pk a value of the indicators. An indicator is a dye that changes colour when it is put into an acid or a base. Most of the indicators are themselves weak acids. The undissociated form of the indicator is a different color than the iogenic form of the indicator.
 Source: bushtuckerchem.wordpress.com
Source: bushtuckerchem.wordpress.com
An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. An indicator gives different colours in acid and base. They are also called ph indicators. One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with.
 Source: chegg.com
Source: chegg.com
One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with. The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. They are also called ph indicators.
 Source: guidancecorner.com
Source: guidancecorner.com
The molecule structure of each indicator is shown below the table. They are usually either weak acids or weak bases. So how do scientists know if a substance is an acid or a base. Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. An indicator is a dye that changes colour when it is put into an acid or a base.
 Source: sciencenotes.org
Source: sciencenotes.org
An indicator gives different colours in acid and base. So how do scientists know if a substance is an acid or a base. They are also called ph indicators. The most widely known indicator is litmus paper which turns red in acidic solutions and blue in basic solutions. The ranges for the color changes are given in the table below the figure together with the corresponding pk a value of the indicators.
 Source: study.com
Source: study.com
However it is easy to make your own. One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with. A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic. An indicator is a dye that changes colour when it is put into an acid or a base. An indicator gives different colours in acid and base.
 Source: 8thgradechemistryasmilan.wordpress.com
Source: 8thgradechemistryasmilan.wordpress.com
They are usually either weak acids or weak bases. An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. An indicator is a dye that changes colour when it is put into an acid or a base. See also pka values of inorganic acids and bases organic nitrogen compound and alcohols and carboxylic acids. An acid base indicator is either a weak acid or weak base that exhibits a color change as the concentration of hydrogen h or hydroxide oh ions changes in an aqueous solution.
 Source: chem.libretexts.org
Source: chem.libretexts.org
A substance which contains an acid is said to be acidic whereas the substance which contains a base is said to be basic. Whereas most dyes do not change color with the amount of acid or base present there are many molecules known as acid base indicators which do respond to a change in the hydrogen ion concentration. So how do scientists know if a substance is an acid or a base. An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. An acid base indicator is a weak acid or a weak base.
 Source: thoughtco.com
Source: thoughtco.com
An acid base indicator is a weak acid or a weak base. An indicator does not change color from pure acid to pure alkaline at specific hydrogen ion concentration but rather color change occurs over a range of hydrogen ion concentrations. Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. They are usually either weak acids or weak bases. Acid base indicators are chemical substances that can give a color change in a reaction medium as a response to a change in ph.
 Source: compoundchem.com
Source: compoundchem.com
One way is using something called an indicator which changes color depending on the ph of the substance it comes in contact with. The ranges for the color changes are given in the table below the figure together with the corresponding pk a value of the indicators. Acid base indicators are most often used in a titration to identify the endpoint of an acid base reaction. See also pka values of inorganic acids and bases organic nitrogen compound and alcohols and carboxylic acids. Most of the indicators are themselves weak acids.
 Source: sites.google.com
Source: sites.google.com
Whereas most dyes do not change color with the amount of acid or base present there are many molecules known as acid base indicators which do respond to a change in the hydrogen ion concentration. These indicators are used to identify the termination of an acid base reaction. Most of the indicators are themselves weak acids. See also pka values of inorganic acids and bases organic nitrogen compound and alcohols and carboxylic acids. They are usually either weak acids or weak bases.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title indicator color change acid or base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.