Iodine reagent test
Iodine Reagent Test. There are enough reagents for 100 tests to be used with a compatible benchtop or portable photometer. This experiment iodine reagent was used to determined the presence of starch in the solution or food. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. Decolouri sation of a weakly alkaline iodine starch solution.
 Iodine Test For Starch Mr Pauller Youtube From youtube.com
Iodine Test For Starch Mr Pauller Youtube From youtube.com
The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Decolouri sation of a weakly alkaline iodine starch solution. 3 2 this test method measures the unsaturation as iodine value by addition of an iodine chlorine reagent. The test can be subjective or quantitative. The amount of reagent absorbed is determined by back titrating the excess reagent and comparing it to a blank determination.
The amount of reagent absorbed is determined by back titrating the excess reagent and comparing it to a blank determination.
The hi93718 01 are reagents for the colorimetric determination of iodine i2. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion. These high quality reagents are manufactured in our state of the art facility and are clearly marked with the lot number and expiration date on each packet for traceability. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. The combination of starch and iodine is intensely blue black. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Decolouri sation of a weakly alkaline iodine starch solution. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. The combination of starch and iodine is intensely blue black.
 Source: alamy.com
Source: alamy.com
The hi93718 01 are reagents for the colorimetric determination of iodine i2. There are enough reagents for 100 tests to be used with a compatible benchtop or portable photometer. The hi93718 01 are reagents for the colorimetric determination of iodine i2. Decolouri sation of a weakly alkaline iodine starch solution. The interaction between starch and the triiodide anion i 3 is the basis for iodometry.
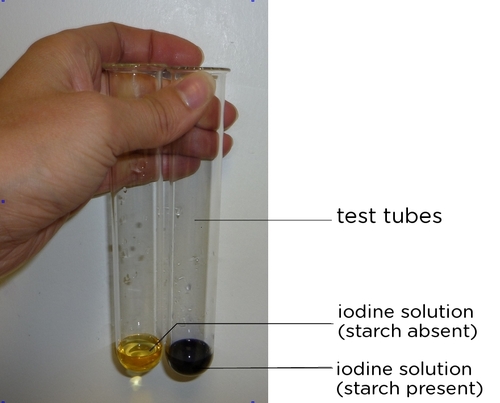 Source: socratic.org
Source: socratic.org
The amount of reagent absorbed is determined by back titrating the excess reagent and comparing it to a blank determination. 3 2 this test method measures the unsaturation as iodine value by addition of an iodine chlorine reagent. These high quality reagents are manufactured in our state of the art facility and are clearly marked with the lot number and expiration date on each packet for traceability. The test can be subjective or quantitative. The combination of starch and iodine is intensely blue black.
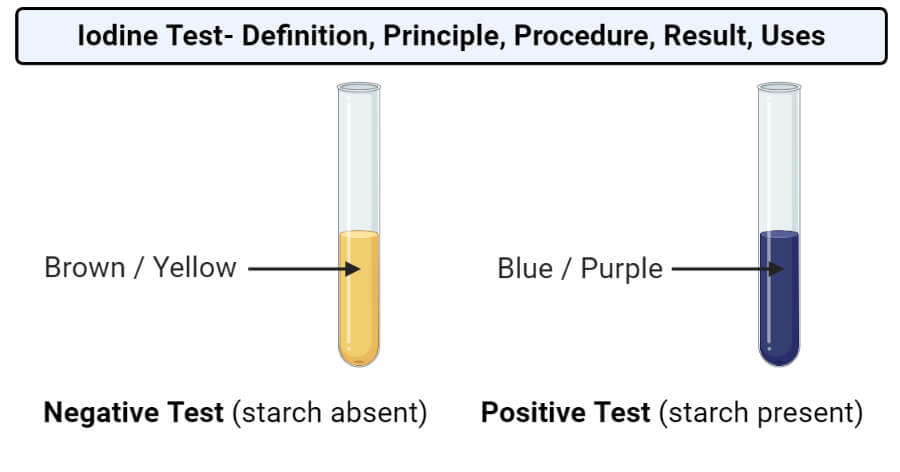 Source: microbenotes.com
Source: microbenotes.com
Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion. The test can be subjective or quantitative. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed. The hi93718 01 are reagents for the colorimetric determination of iodine i2. The iodine test is utilized to test for the presence of starch.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed. Decolouri sation of a weakly alkaline iodine starch solution. This experiment iodine reagent was used to determined the presence of starch in the solution or food. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed.
 Source: dalconenvironmental.com.au
Source: dalconenvironmental.com.au
The complexity of the molecular. The iodine test is utilized to test for the presence of starch. The test can be subjective or quantitative. The combination of starch and iodine is intensely blue black. Iodine on its own is insoluble in water.
 Source: nku.edu
Source: nku.edu
The complexity of the molecular. The combination of starch and iodine is intensely blue black. This experiment iodine reagent was used to determined the presence of starch in the solution or food. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine.
 Source: michelle1004.weebly.com
Source: michelle1004.weebly.com
The test can be subjective or quantitative. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. There are enough reagents for 100 tests to be used with a compatible benchtop or portable photometer. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed.

Decolouri sation of a weakly alkaline iodine starch solution. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion. The hi93718 01 are reagents for the colorimetric determination of iodine i2. Iodine on its own is insoluble in water. This experiment iodine reagent was used to determined the presence of starch in the solution or food.
 Source: biology4alevel.blogspot.com
Source: biology4alevel.blogspot.com
The combination of starch and iodine is intensely blue black. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion. The test is based on several coupled equilibria and h ypoiodous acid is the oxidizing agent. The combination of starch and iodine is intensely blue black. The iodine test is utilized to test for the presence of starch.
 Source: sites.google.com
Source: sites.google.com
The hi93718 01 are reagents for the colorimetric determination of iodine i2. The complexity of the molecular. The test is based on several coupled equilibria and h ypoiodous acid is the oxidizing agent. Iodine on its own is insoluble in water. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion.
 Source: nerdynaut.com
Source: nerdynaut.com
The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Iodine on its own is insoluble in water. Decolouri sation of a weakly alkaline iodine starch solution. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring.
 Source: satuluqehujycozu.usagiftsshops.com
Source: satuluqehujycozu.usagiftsshops.com
3 2 this test method measures the unsaturation as iodine value by addition of an iodine chlorine reagent. The combination of starch and iodine is intensely blue black. Decolouri sation of a weakly alkaline iodine starch solution. The test can be subjective or quantitative. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed.
 Source: youtube.com
Source: youtube.com
Decolouri sation of a weakly alkaline iodine starch solution. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a pentaiodide ion. Iodine on its own is insoluble in water.
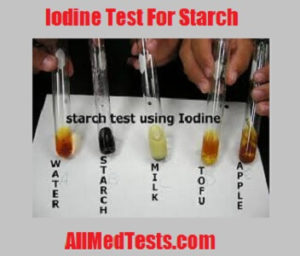 Source: allmedtests.com
Source: allmedtests.com
The complexity of the molecular. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. The test can be subjective or quantitative. When iodine reagent added in the starch presence solution the iodine ion will integrate in the polysaccharide chain and result in a dark blue colour the positive result showed. These high quality reagents are manufactured in our state of the art facility and are clearly marked with the lot number and expiration date on each packet for traceability.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title iodine reagent test by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







