Is copper an alkali metal
Is Copper An Alkali Metal. Copper is not an alkaline earth metal it is a transition metal also known as a coinage metal. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was. Alkali metal alkali metal chemical properties. All the alkali metals when heated with oxygen form different types of oxides for example lithium forms lithium oxide sodium forms sodium peroxide while k rb and cs form their respective superoxides where m k rb or cs.
 Transition Metals Periodic Table Groups From periodictablegroups.wordpress.com
Transition Metals Periodic Table Groups From periodictablegroups.wordpress.com
It is one of only three metals that are clearly coloured the other two being copper and gold. Strong alkaline solutions do not affect metals of the copper subgroup copper silver and gold and the iron subgroup iron cobalt and nickel and cadmium magnesium the rare earth metals thallium thorium and the platinum metals. Copper is not an alkaline earth metal it is a transition metal also known as a coinage metal. The mobilities of the alkali metal ions in aqueous solution are. Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device.
And smelt the copper minerals to produce copper metal.
Molybdenum tungsten vanadium and tantalum are stable in alkaline solutions at room temperature. Alkali metal alkali metal chemical properties. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device. It is one of only three metals that are clearly coloured the other two being copper and gold. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint.
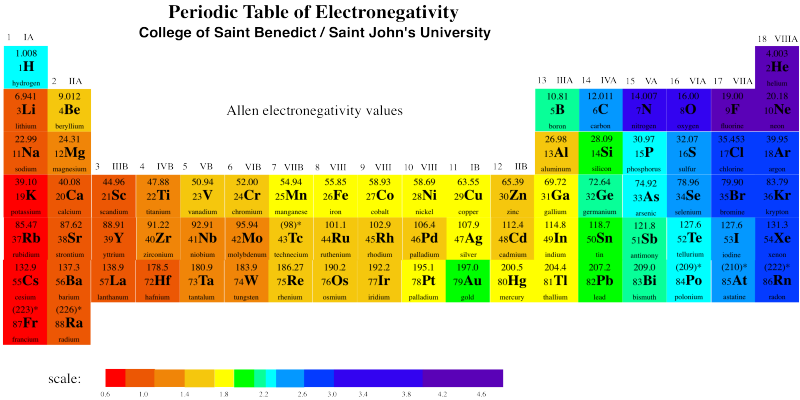 Source: employees.csbsju.edu
Source: employees.csbsju.edu
All the alkali metals when heated with oxygen form different types of oxides for example lithium forms lithium oxide sodium forms sodium peroxide while k rb and cs form their respective superoxides where m k rb or cs. It is one of only three metals that are clearly coloured the other two being copper and gold. Alkali metal alkali metal chemical properties. And smelt the copper minerals to produce copper metal. The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint.
 Source: periodictablegroups.wordpress.com
Source: periodictablegroups.wordpress.com
The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was. Cu is the chemical symbol for copper. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Cu is the chemical symbol for copper. Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. C eo for m 2 aq 2e m s where m ca sr or ba is nearly constant. Li na k rb cs b lithium is the only alkali metal to form a nitride directly. All the alkali metals when heated with oxygen form different types of oxides for example lithium forms lithium oxide sodium forms sodium peroxide while k rb and cs form their respective superoxides where m k rb or cs.
 Source: sciencedirect.com
Source: sciencedirect.com
They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device. Li na k rb cs b lithium is the only alkali metal to form a nitride directly.
 Source: researchgate.net
Source: researchgate.net
In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. Cu is the chemical symbol for copper. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device. Alkali metal alkali metal chemical properties. And smelt the copper minerals to produce copper metal.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The mobilities of the alkali metal ions in aqueous solution are. This review of the unique effects due to different alkali elements shows the impact on the absorber layers in various ways. The mobilities of the alkali metal ions in aqueous solution are. The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The mobilities of the alkali metal ions in aqueous solution are. Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group. The increasing stability of peroxides and superoxides of alkali metals from li to cs is due to stabilisation of larger. Cu is the chemical symbol for copper.
 Source: researchgate.net
Source: researchgate.net
A the alkali metal ions are highly hydrated. The stable alkali metals are all silver coloured metals except for caesium which has a pale golden tint. Cu is the chemical symbol for copper. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. The mobilities of the alkali metal ions in aqueous solution are.
 Source: en.wikipedia.org
Source: en.wikipedia.org
And smelt the copper minerals to produce copper metal. And smelt the copper minerals to produce copper metal. Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. Copper is not an alkaline earth metal it is a transition metal also known as a coinage metal. The smaller the size of the ion the.
 Source: britannica.com
Source: britannica.com
Cu is the chemical symbol for copper. Cu is the chemical symbol for copper. A the alkali metal ions are highly hydrated. The smaller the size of the ion the. In its chemical reactivity lithium more closely resembles group 2 iia of the periodic table than it does the other metals of its own group.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
A the alkali metal ions are highly hydrated. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was. Cu is the chemical symbol for copper. It is one of only three metals that are clearly coloured the other two being copper and gold. It is less reactive than the other alkali metals with water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. It is one of only three metals that are clearly coloured the other two being copper and gold. This review of the unique effects due to different alkali elements shows the impact on the absorber layers in various ways. In alkali metal percent of earths crust the other alkali metals are considerably more rare with rubidium lithium and cesium respectively forming and percent of earths crust francium a natural radioactive isotope is very rare and was. Copper is not an alkaline earth metal it is a transition metal also known as a coinage metal.
 Source: pinterest.ph
Source: pinterest.ph
They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Strong alkaline solutions do not affect metals of the copper subgroup copper silver and gold and the iron subgroup iron cobalt and nickel and cadmium magnesium the rare earth metals thallium thorium and the platinum metals. The incorporation of alkali metals has provided tremendous cigse solar cells improvement with a high record efficiency 22 6 when utilizing heavy alkali metal treatments on the device. This review of the unique effects due to different alkali elements shows the impact on the absorber layers in various ways. The mobilities of the alkali metal ions in aqueous solution are.
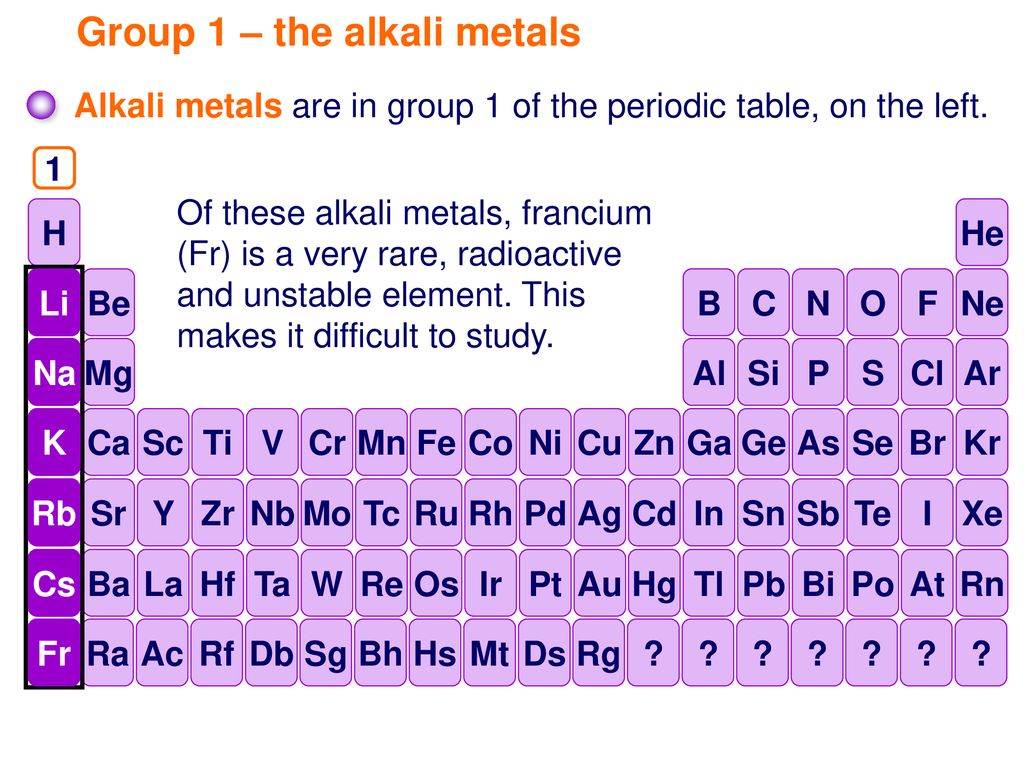 Source: slideplayer.com
Source: slideplayer.com
The increasing stability of peroxides and superoxides of alkali metals from li to cs is due to stabilisation of larger. All the alkali metals when heated with oxygen form different types of oxides for example lithium forms lithium oxide sodium forms sodium peroxide while k rb and cs form their respective superoxides where m k rb or cs. Cu is the chemical symbol for copper. Li na k rb cs b lithium is the only alkali metal to form a nitride directly. Molybdenum tungsten vanadium and tantalum are stable in alkaline solutions at room temperature.
 Source: slideshare.net
Source: slideshare.net
Since the alkali metals are the most electropositive the least electronegative of elements they react with a great variety of nonmetals. Strong alkaline solutions do not affect metals of the copper subgroup copper silver and gold and the iron subgroup iron cobalt and nickel and cadmium magnesium the rare earth metals thallium thorium and the platinum metals. The mobilities of the alkali metal ions in aqueous solution are. This review of the unique effects due to different alkali elements shows the impact on the absorber layers in various ways. It is less reactive than the other alkali metals with water.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is copper an alkali metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






