Least reactive elements on the periodic table
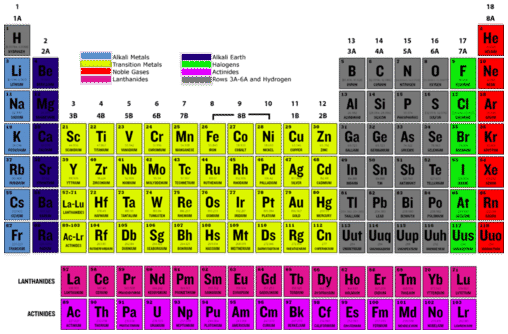
Least Reactive Elements On The Periodic Table. Since they have 8 electrons in the outer shell and because the maximum number of electrons in the outer shell is 8 electrons then the element doesn t need any extra electrons nor does it need to get rid of it. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements. Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shell. Neon has an atomic number of 10.
 6 11 Noble Gases Chemistry Libretexts From chem.libretexts.org
6 11 Noble Gases Chemistry Libretexts From chem.libretexts.org
It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air. Potassium k is an alkali metal placed under sodium and over rubidium and is the first element of period 4. Mg3n2 magnesium nitride 41. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity. There is published data to help you determine relative. At standard temperature and pressure lithium is a soft silver white highly reactive metal.
Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shell.
Most reactive na least ag34. Most reactive na least ag34. These are the elements of group 18 and they have full valence shells. There is published data to help you determine relative. They are not interested in. It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air.
 Source: compoundchem.com
Source: compoundchem.com
As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. Most reactive na least ag34. These are the elements of group 18 and they have full valence shells. The least reactive elements in the periodic table are the noble gases or sometimes the inert gases. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements. Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shell. Lithium is the first alkali metal in the periodic table and the first metal of any kind in the periodic table. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. Since they have 8 electrons in the outer shell and because the maximum number of electrons in the outer shell is 8 electrons then the element doesn t need any extra electrons nor does it need to get rid of it.
 Source: theperiodictablenick.weebly.com
Source: theperiodictablenick.weebly.com
Neon has an atomic number of 10. Neon has an atomic number of 10. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. Neon is the least reactive element on the periodic table. It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air.
Source: quora.com
The least reactive elements in the periodic table are the noble gases or sometimes the inert gases. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. Mg3n2 magnesium nitride 41. They are not interested in. Most reactive na least ag34.
 Source: chem.libretexts.org
Source: chem.libretexts.org
With a density of 0 564 g cm 3 lithium is the lightest metal and the least dense solid element. Lithium is the first alkali metal in the periodic table and the first metal of any kind in the periodic table. They are not interested in. There is published data to help you determine relative. It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air.
 Source: slideplayer.com
Source: slideplayer.com
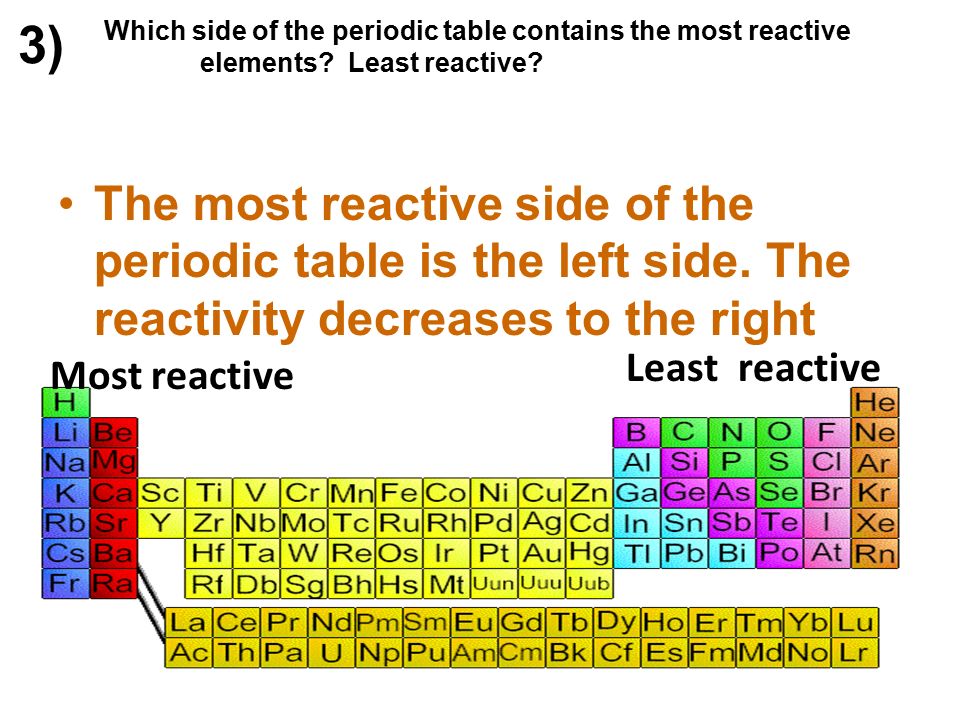
Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity. Most reactive na least ag34. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. The right most column group 8 or the noble gases is the least reactive. Neon has an atomic number of 10.
 Source: scienceabc.com
Source: scienceabc.com
They are not interested in. At standard temperature and pressure lithium is a soft silver white highly reactive metal. Lithium is the first alkali metal in the periodic table and the first metal of any kind in the periodic table. With a density of 0 564 g cm 3 lithium is the lightest metal and the least dense solid element. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state.
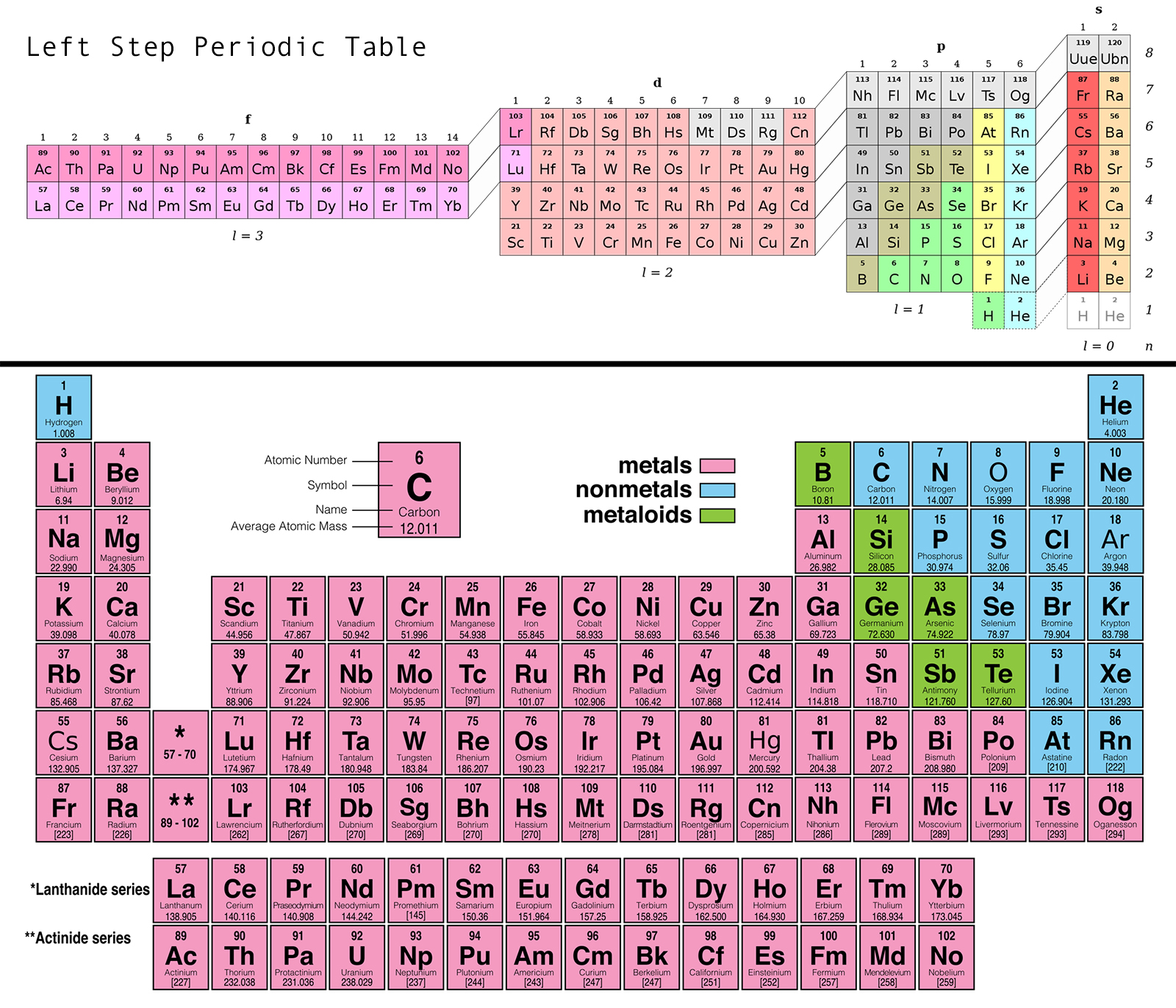 Source: dailybruin.com
Source: dailybruin.com
The right most column group 8 or the noble gases is the least reactive. Mg3n2 magnesium nitride 41. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shell. With a density of 0 564 g cm 3 lithium is the lightest metal and the least dense solid element.
 Source: pinterest.co.uk
Source: pinterest.co.uk
Mg3n2 magnesium nitride 41. It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. At standard temperature and pressure lithium is a soft silver white highly reactive metal. Neon is the least reactive element on the periodic table.
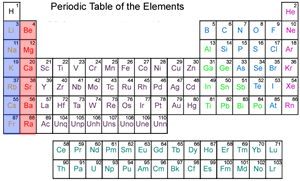 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The right most column group 8 or the noble gases is the least reactive. It is one of the most reactive elements in the periodic table therefore usually only found in compounds it tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air. Mg3n2 magnesium nitride 41. The right most column group 8 or the noble gases is the least reactive. They are not interested in.
Source: quora.com
Mg3n2 magnesium nitride 41. At standard temperature and pressure lithium is a soft silver white highly reactive metal. They are not interested in. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements. The right most column group 8 or the noble gases is the least reactive.
 Source: ck12.org
Source: ck12.org
Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shell. Neon is the least reactive element on the periodic table. These are the elements of group 18 and they have full valence shells. Lithium is the first alkali metal in the periodic table and the first metal of any kind in the periodic table. They are not interested in.
 Source: toppr.com
Source: toppr.com
These are the elements of group 18 and they have full valence shells. Most reactive na least ag34. Mg3n2 magnesium nitride 41. The right most column group 8 or the noble gases is the least reactive. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity.
 Source: quora.com
Source: quora.com
There is published data to help you determine relative. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. These are the elements of group 18 and they have full valence shells. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state.
 Source: britannica.com
Source: britannica.com
As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. With a density of 0 564 g cm 3 lithium is the lightest metal and the least dense solid element. Potassium k is an alkali metal placed under sodium and over rubidium and is the first element of period 4. They are not interested in. Since they have 8 electrons in the outer shell and because the maximum number of electrons in the outer shell is 8 electrons then the element doesn t need any extra electrons nor does it need to get rid of it.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title least reactive elements on the periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.