List of unreactive metals
List Of Unreactive Metals. All of the alkaline earth metal atoms have a 2 oxidation state. Finally most metal elements lose electrons during reactions. However different metals have different reactivities towards oxygen unreactive metals such as gold and platinum do not readily form oxides when exposed to air. One way to make a salt is to react a metal with an acid.
 Reactivity Series Of Metals Get The Chart Significance And Much More From toppr.com
Reactivity Series Of Metals Get The Chart Significance And Much More From toppr.com
The reactivity series of metals is a chart listing metals in order of decreasing reactivity. This means they do not easily take part in chemical reactions. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. For example platinum does not react with oxygen in the air even if it is heated in a bunsen burner. It is used to summarize information about the reactions of metals with acids and water single displacement reactions and the extraction of metals from their ores. All of the alkaline earth metal atoms have a 2 oxidation state.
List of metals 5 this entry was posted on september 3 2014 by todd helmenstine updated on april 9 2020 the highlighted elements are considered the metal elements.
However different metals have different reactivities towards oxygen unreactive metals such as gold and platinum do not readily form oxides when exposed to air. The alkaline earth metals are found in group iia of the periodic table which is the second column of elements. The largest group of elements on the periodic table is that of the transition metals which is found in the middle of the table. This means they do not easily take part in chemical reactions. In general the more reactive a metal is. Also the two rows of elements below the main body of the periodic table the lanthanides and actinides are special subsets of these metals.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
The alkaline earth metals are found in group iia of the periodic table which is the second column of elements. This is hydrogen that when exposed to either extremely high or extremely low temperatures can display some of these common properties. One such reaction is the reaction of. One way to make a salt is to react a metal with an acid. For example platinum does not react with oxygen in the air even if it is heated in a bunsen burner.
 Source: pinterest.co.uk
Source: pinterest.co.uk
Potassium is the most reactive metal in this list and gold is the least reactive. August 31 2014 chemlegin. Salient features the metals at the top of the reactivity series are powerful reducing agents since they are easily oxidized. In chemistry a reactivity series or activity series is an empirical calculated and structurally analytical progression of a series of metals arranged by their reactivity from highest to lowest. Like the alkali metals these elements are found in compounds rather than pure form.
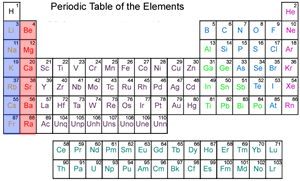 Source: dynamicscience.com.au
Source: dynamicscience.com.au
At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. For example platinum does not react with oxygen in the air even if it is heated in a bunsen burner. This is hydrogen that when exposed to either extremely high or extremely low temperatures can display some of these common properties. Some metals are very unreactive. List of metals 5 this entry was posted on september 3 2014 by todd helmenstine updated on april 9 2020 the highlighted elements are considered the metal elements.
 Source: quora.com
Source: quora.com
Salient features the metals at the top of the reactivity series are powerful reducing agents since they are easily oxidized. The more vigorously it reacts with other substances the more. Finally most metal elements lose electrons during reactions. This means they do not easily take part in chemical reactions. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals.
Source: quora.com
Like the alkali metals these elements are found in compounds rather than pure form. One such reaction is the reaction of. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. List of metals 5 this entry was posted on september 3 2014 by todd helmenstine updated on april 9 2020 the highlighted elements are considered the metal elements. For example platinum does not react with oxygen in the air even if it is heated in a bunsen burner.
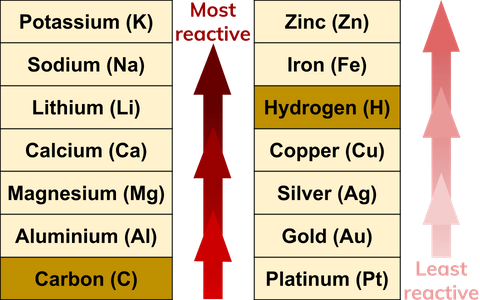 Source: senecalearning.com
Source: senecalearning.com
All of the alkaline earth metal atoms have a 2 oxidation state. This is hydrogen that when exposed to either extremely high or extremely low temperatures can display some of these common properties. Potassium is the most reactive metal in this list and gold is the least reactive. Like the alkali metals these elements are found in compounds rather than pure form. Some metals are very unreactive.
 Source: toppr.com
Source: toppr.com
In general the more reactive a metal is. There is one non metal element that can sometimes act as a metal. One such reaction is the reaction of. Like the alkali metals these elements are found in compounds rather than pure form. The complete list of metals.
 Source: smackslide.com
Source: smackslide.com
In general the more reactive a metal is. Like the alkali metals these elements are found in compounds rather than pure form. The complete list of metals. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. Some metals are very unreactive.
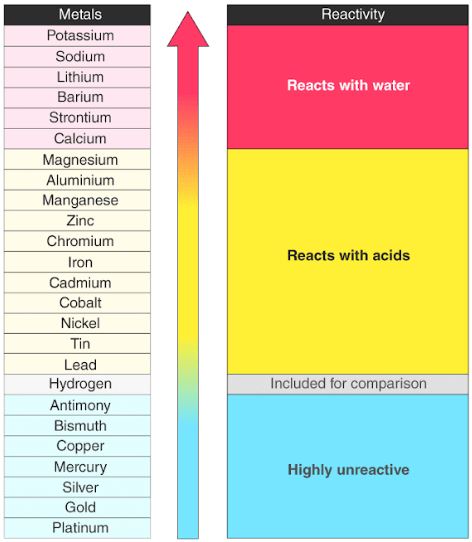 Source: byjus.com
Source: byjus.com
Alkaline earth metals. The largest group of elements on the periodic table is that of the transition metals which is found in the middle of the table. However different metals have different reactivities towards oxygen unreactive metals such as gold and platinum do not readily form oxides when exposed to air. Potassium is the most reactive metal in this list and gold is the least reactive. One way to make a salt is to react a metal with an acid.
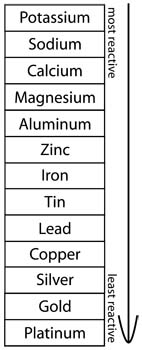 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
However different metals have different reactivities towards oxygen unreactive metals such as gold and platinum do not readily form oxides when exposed to air. One such reaction is the reaction of. There is one non metal element that can sometimes act as a metal. Potassium is the most reactive metal in this list and gold is the least reactive. It is used to summarize information about the reactions of metals with acids and water single displacement reactions and the extraction of metals from their ores.
 Source: quora.com
Source: quora.com
The largest group of elements on the periodic table is that of the transition metals which is found in the middle of the table. The alkaline earth metals are found in group iia of the periodic table which is the second column of elements. Some metals are very unreactive. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. Finally most metal elements lose electrons during reactions.
 Source: chemlegin.wordpress.com
Source: chemlegin.wordpress.com
There is one non metal element that can sometimes act as a metal. All of the alkaline earth metal atoms have a 2 oxidation state. The complete list of metals. One way to make a salt is to react a metal with an acid. Some metals are very unreactive.
 Source: quora.com
Source: quora.com
List of metals 5 this entry was posted on september 3 2014 by todd helmenstine updated on april 9 2020 the highlighted elements are considered the metal elements. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. Like the alkali metals these elements are found in compounds rather than pure form. Some metals are very unreactive. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals.
 Source: chemlegin.wordpress.com
Source: chemlegin.wordpress.com
Potassium is the most reactive metal in this list and gold is the least reactive. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. Potassium is the most reactive metal in this list and gold is the least reactive. At school students frequently carry out reactions on a test tube scale to illustrate the reactivity series of metals. In general the more reactive a metal is.
 Source: mammothmemory.net
Source: mammothmemory.net
The alkaline earth metals are found in group iia of the periodic table which is the second column of elements. One way to make a salt is to react a metal with an acid. For example platinum does not react with oxygen in the air even if it is heated in a bunsen burner. The alkaline earth metals are found in group iia of the periodic table which is the second column of elements. Potassium is the most reactive metal in this list and gold is the least reactive.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title list of unreactive metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







