Lugol s iodine test
Lugol S Iodine Test. In a lab lugol s solution is typically used as an indicator for the presence of starch in a solution. Lugol s solution also called lugol s iodine is a solution of elemental iodine and potassium iodide in water that generally causes a solution containing starch to turn deep blue. The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5. Lugols formula iodine is available in liquid tablet and capsule forms.
 Lugol S Iodine Solution From dalconenvironmental.com.au
Lugol S Iodine Solution From dalconenvironmental.com.au
Lugol s iodine solution is a starch indicator chemical reagent and biological stain. Iodine on its own is insoluble in water. The term lugols iodine refers to the iodine formula first discovered by dr. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. In a lab lugol s solution is typically used as an indicator for the presence of starch in a solution. For example if iodine is added to a peeled potato then it will turn black.
The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5.
Lugols formula iodine is available in liquid tablet and capsule forms. Lugol s iodine solution is a starch indicator chemical reagent and biological stain. The term lugols iodine refers to the iodine formula first discovered by dr. Lugol s iodine yields a blue black color in the presence of starch. The interaction between starch and the triiodide anion i 3 is the basis for iodometry. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring.
 Source: nku.edu
Source: nku.edu
This might be because the iodine in iodophor is released slower compared to the iodine in lugol s iodine but both solutions are still equally well suited for starch testing in brewing. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine the combination of starch and iodine is intensely blue black. The test solution remains the characteristic brown yellow of the reagent. The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5. This chemical stains carbohydrates in plant and animal specimens brown or blue black and stains glycogen red.
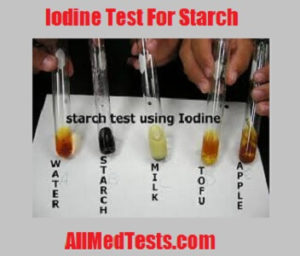 Source: allmedtests.com
Source: allmedtests.com
Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a. It is thought that starch and glycogen form helical coils. The term lugols iodine refers to the iodine formula first discovered by dr. The iodine test is utilized to test for the presence of starch. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a.
 Source: slideplayer.com
Source: slideplayer.com
Lugol who created the formula in 1829 consisting of 85 distilled water 10 potassium iodide and 5 iodine. This might be because the iodine in iodophor is released slower compared to the iodine in lugol s iodine but both solutions are still equally well suited for starch testing in brewing. Other polysaccharides and monosaccharides yield no color change. Lugol s iodine solution is a starch indicator chemical reagent and biological stain. The interaction between starch and the triiodide anion i 3 is the basis for iodometry.
 Source: slideplayer.com
Source: slideplayer.com
The iodine test is utilized to test for the presence of starch. This 30 ml bottle is an aqueous solution of 1 8 iodine and 3 0 potassium iodide. A starch test solution made from lugol s iodine tends to give a clearer reaction with starch than one prepared from iodophor. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. This video shows how lugol s iodine is used to test a liquid solution for the presence of starch.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
Other polysaccharides and monosaccharides yield no color change. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine the combination of starch and iodine is intensely blue black. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. Lugols formula iodine is available in liquid tablet and capsule forms. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide.
 Source: quizlet.com
Source: quizlet.com
Lugol s iodine yields a blue black color in the presence of starch. This 30 ml bottle is an aqueous solution of 1 8 iodine and 3 0 potassium iodide. The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5. Glycogen reacts with lugol s reagent to give a brown blue color. The tube on the left contains only water and the test prod.
 Source: pinterest.com
Source: pinterest.com
It is possible to distinguish starch from glucose and other carbohydrates using this iodine solution test. The test solution remains the characteristic brown yellow of the reagent. This might be because the iodine in iodophor is released slower compared to the iodine in lugol s iodine but both solutions are still equally well suited for starch testing in brewing. Lugol s solution also called lugol s iodine is a solution of elemental iodine and potassium iodide in water that generally causes a solution containing starch to turn deep blue. Benedict s reagent can be used to test for glucose.
 Source: sites.google.com
Source: sites.google.com
In a lab lugol s solution is typically used as an indicator for the presence of starch in a solution. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide. Lugol s solution also called lugol s iodine is a solution of elemental iodine and potassium iodide in water that generally causes a solution containing starch to turn deep blue. This chemical stains carbohydrates in plant and animal specimens brown or blue black and stains glycogen red. The iodine test is utilized to test for the presence of starch.
 Source: nku.edu
Source: nku.edu
The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine the combination of starch and iodine is intensely blue black. This 30 ml bottle is an aqueous solution of 1 8 iodine and 3 0 potassium iodide. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion which further reacts with an iodine molecule to form a. The interaction between starch and the triiodide anion i 3 is the basis for iodometry. The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5.
 Source: slideshare.net
Source: slideshare.net
The interaction between starch and the triiodide anion i 3 is the basis for iodometry. Lugol s solution also called lugol s iodine is a solution of elemental iodine and potassium iodide in water that generally causes a solution containing starch to turn deep blue. It is possible to distinguish starch from glucose and other carbohydrates using this iodine solution test. This video shows how lugol s iodine is used to test a liquid solution for the presence of starch. The term lugols iodine refers to the iodine formula first discovered by dr.
 Source: homesciencetools.com
Source: homesciencetools.com
The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine the combination of starch and iodine is intensely blue black. Lugol s iodine yields a blue black color in the presence of starch. In the presence of starch iodine turns a blue black colour. Lugol s iodine solution is a starch indicator chemical reagent and biological stain. The iodine test is utilized to test for the presence of starch.
 Source: alevelbiologynotes.com
Source: alevelbiologynotes.com
In the presence of starch iodine turns a blue black colour. The test solution remains the characteristic brown yellow of the reagent. This chemical stains carbohydrates in plant and animal specimens brown or blue black and stains glycogen red. In a lab lugol s solution is typically used as an indicator for the presence of starch in a solution. Benedict s reagent can be used to test for glucose.
 Source: youtube.com
Source: youtube.com
Lugol s iodine yields a blue black color in the presence of starch. This chemical stains carbohydrates in plant and animal specimens brown or blue black and stains glycogen red. It is possible to distinguish starch from glucose and other carbohydrates using this iodine solution test. In the presence of starch iodine turns a blue black colour. The reagent used in the iodine test is lugol s iodine which is an aqueous solution of elemental iodine and potassium iodide.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The iodine test is utilized to test for the presence of starch. This 30 ml bottle is an aqueous solution of 1 8 iodine and 3 0 potassium iodide. This might be because the iodine in iodophor is released slower compared to the iodine in lugol s iodine but both solutions are still equally well suited for starch testing in brewing. This video shows how lugol s iodine is used to test a liquid solution for the presence of starch. It is possible to distinguish starch from glucose and other carbohydrates using this iodine solution test.
 Source: dalconenvironmental.com.au
Source: dalconenvironmental.com.au
This 30 ml bottle is an aqueous solution of 1 8 iodine and 3 0 potassium iodide. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. The coloring can be differentiated outwardly with centralizations of iodine as low as 2 10 5. Benedict s reagent can be used to test for glucose. The interaction between starch and the triiodide anion i 3 is the basis for iodometry.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lugol s iodine test by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.