Metals that corrode easily
Metals That Corrode Easily. Copper and silver did not corrode at all. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. Increasing t speeds up oxidation reduction reactions. The galvanic series ranks the reactivity of metals in seawater.
 Why Metal Roofs Corrode What Causes Rust From ddcoatings.co.uk
Why Metal Roofs Corrode What Causes Rust From ddcoatings.co.uk
The most active metals which tend to lose electrons easily such as magnesium and aluminum corrode easily. Increasing t speeds up oxidation reduction reactions. Metals with a more negative standard electrode potential are more likely to corrode relative to other metals. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper. Technically only iron and alloys that contain iron can rust.
Copper and silver did not corrode at all.
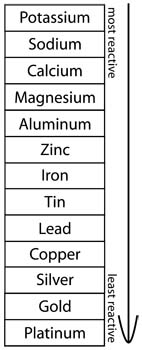
For instance a less noble metal like zinc will dissolve and form the anode whereas the more noble metal such as copper will act as the cathode. Copper and silver did not corrode at all. You ll test five metals silver steel zinc copper and aluminum to see which corrodes fastest in water and in salt water. The galvanic series ranks the reactivity of metals in seawater. Here are a few of the most common metals and how they stand up against rust and corrosion. Technically only iron and alloys that contain iron can rust.
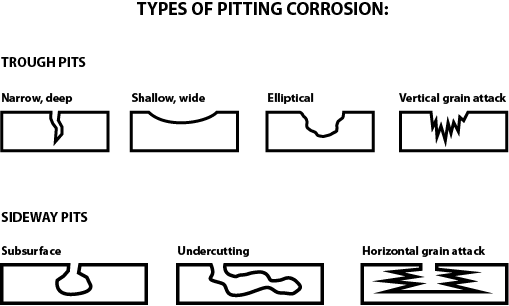 Source: gibsonstainless.com
Source: gibsonstainless.com
Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish. Here are a few of the most common metals and how they stand up against rust and corrosion. The most stable metals those which do not lose electrons easily such as gold and silver do not corrode easily. Copper and silver did not corrode at all. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture.
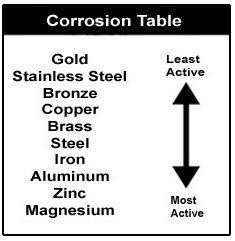
The claim was made based off the evidence and research provided. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. The galvanic series ranks the reactivity of metals in seawater. The claim was made based off the evidence and research provided. Even though it looked like iron was fastest to corrode it was really aluminum that was fastest to corrode.
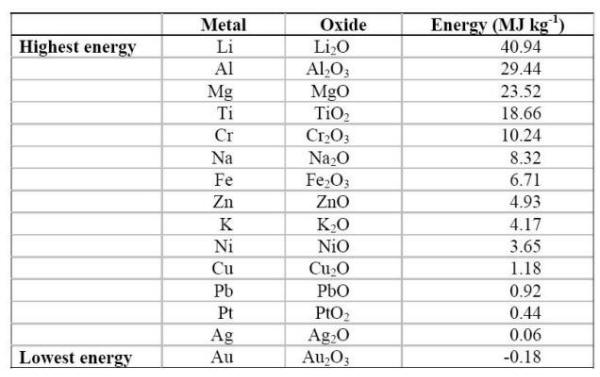 Source: corrosion-doctors.org
Source: corrosion-doctors.org
Technically only iron and alloys that contain iron can rust. The most active metals which tend to lose electrons easily such as magnesium and aluminum corrode easily. All metals will rust but at differing rates. Rust is also seen as tarnish on some metals. The claim was made based off the evidence and research provided.
 Source: xapps.xyleminc.com
Source: xapps.xyleminc.com
Gold and platinum oxides are very rare because not only will it take many years for these metals to rust but the amount will be very insignificant. Other metals including precious metals like gold and silver can corrode in a similar way. Metals with a more negative standard electrode potential are more likely to corrode relative to other metals. Increasing t speeds up oxidation reduction reactions. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper.

Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish. The most active metals which tend to lose electrons easily such as magnesium and aluminum corrode easily. Even though it looked like iron was fastest to corrode it was really aluminum that was fastest to corrode. Here are a few of the most common metals and how they stand up against rust and corrosion. Metals with a more negative standard electrode potential are more likely to corrode relative to other metals.
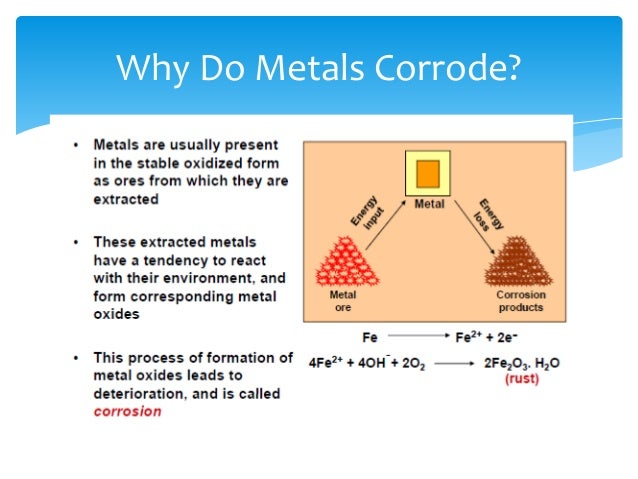 Source: slideshare.net
Source: slideshare.net
Gold and platinum oxides are very rare because not only will it take many years for these metals to rust but the amount will be very insignificant. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper. Using metals which form a protective oxide layer reducing t. For instance a less noble metal like zinc will dissolve and form the anode whereas the more noble metal such as copper will act as the cathode. Copper and silver did not corrode at all.
 Source: slideplayer.com
Source: slideplayer.com
The most active metals which tend to lose electrons easily such as magnesium and aluminum corrode easily. Thus the anode metal is made to corrode or dissolve continuously by the galvanic action. Corrosion may be controlled by. Using metals which form a protective oxide layer reducing t. Increasing t speeds up oxidation reduction reactions.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
Metals with a more negative standard electrode potential are more likely to corrode relative to other metals. Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish. You ll test five metals silver steel zinc copper and aluminum to see which corrodes fastest in water and in salt water. When this happens metal ions flow from the more active metal to the less active metal causing the more active metal to corrode at an accelerated rate and the less active metal to corrode at a slower rate. Gold and platinum oxides are very rare because not only will it take many years for these metals to rust but the amount will be very insignificant.
 Source: ddcoatings.co.uk
Source: ddcoatings.co.uk
Copper and silver did not corrode at all. Other metals including precious metals like gold and silver can corrode in a similar way. Metals with a more negative standard electrode potential are more likely to corrode relative to other metals. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. Technically only iron and alloys that contain iron can rust.
 Source: xapps.xyleminc.com
Source: xapps.xyleminc.com
The galvanic series ranks the reactivity of metals in seawater. The most active metals which tend to lose electrons easily such as magnesium and aluminum corrode easily. You ll test five metals silver steel zinc copper and aluminum to see which corrodes fastest in water and in salt water. Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish. Here are a few of the most common metals and how they stand up against rust and corrosion.
 Source: ddcoatings.co.uk
Source: ddcoatings.co.uk
Gold and platinum oxides are very rare because not only will it take many years for these metals to rust but the amount will be very insignificant. When you ve finished you ll have a better understanding of corrosion the process of oxidation and the properties of different metals. Using metals which form a protective oxide layer reducing t. What sets certain metals apart is the duration of time it takes for them to begin rusting or corroding. Increasing t speeds up oxidation reduction reactions.
 Source: larapedia.com
Source: larapedia.com
The most stable metals those which do not lose electrons easily such as gold and silver do not corrode easily. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. Thus the anode metal is made to corrode or dissolve continuously by the galvanic action. Even though it looked like iron was fastest to corrode it was really aluminum that was fastest to corrode. Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish.
 Source: thoughtco.com
Source: thoughtco.com
Aluminum was the fastest to corrode followed up by zinc iron copper and silver respectively. Corrosion may be controlled by. Corrosion is a chemical reaction that takes place between metal and oxygen in the presence of moisture. Other metals including precious metals like gold and silver can corrode in a similar way. In practical terms this means that corrosion will develop on the more active metal at the point of contact between the two metals.
 Source: tampasteel.com
Source: tampasteel.com
Copper oxidizes over time to form a green patina which actually protects the metal from further corrosion. Aluminum was the fastest to corrode followed up by zinc iron copper and silver respectively. Other metals including precious metals like gold and silver can corrode in a similar way. Even though it looked like iron was fastest to corrode it was really aluminum that was fastest to corrode. Increasing t speeds up oxidation reduction reactions.
 Source: docbrown.info
Source: docbrown.info
What sets certain metals apart is the duration of time it takes for them to begin rusting or corroding. For instance a less noble metal like zinc will dissolve and form the anode whereas the more noble metal such as copper will act as the cathode. Technically only iron and alloys that contain iron can rust. Rust is also seen as tarnish on some metals. Copper oxidizes over time to form a green patina which actually protects the metal from further corrosion.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title metals that corrode easily by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






