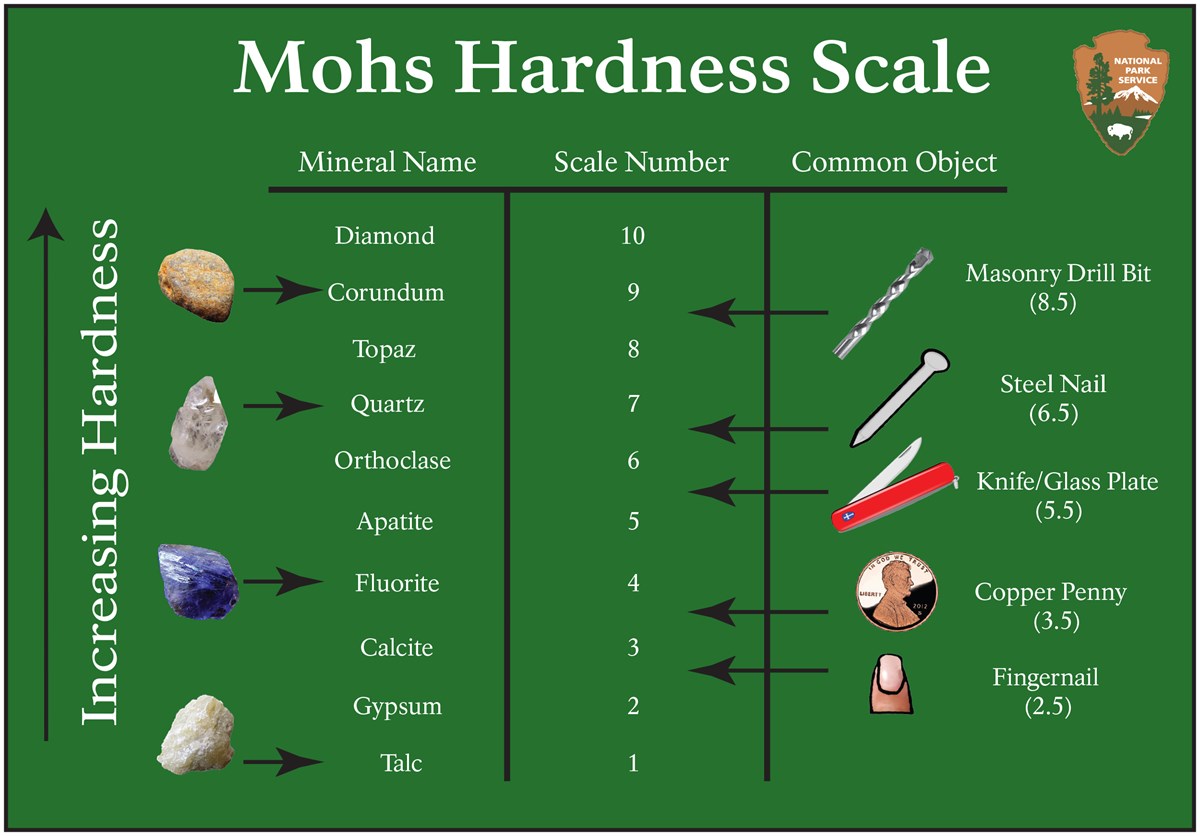
Molecular compound formula
Molecular Compound Formula. Sulfuric acid 98 072 g mol. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Na 3 po 4. The molecular formula is an important piece of information for any chemical compound.
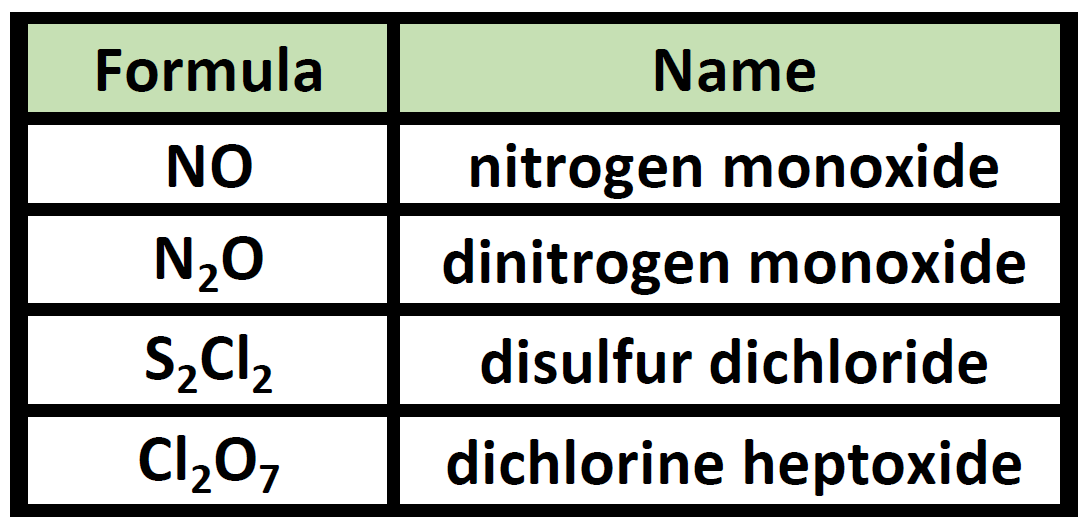 Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry From wou.edu
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry From wou.edu
Molecular formula tells the exact number and type of atoms in a single molecule of a compound. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. H 3 po 4. In molecular formulae the elements are denoted by their respective symbols as in the periodic table and the number of atoms of each element in the molecule is written in subscript. Ch 3 coo 5. H 2 so 4.
H 3 po 4.
Molecular formula or chemical formula of a compound is the representation of the types of atoms and their ratios present in that compound. Chemical elements periodic table compound name formula search moles to grams calculator common compounds list chemical equation balancer complete list of acids complete list of bases molar to mass concentration converter molar mass calculator cations anions list dilution calculator molarity calculator. The molecular formula is an important piece of information for any chemical compound. Molecular formula tells the exact number and type of atoms in a single molecule of a compound. A molecule of octane which is a component of gasoline contains 8 atoms of carbon and 18 atoms of hydrogen. The chemical formula will always be some integer multiple n of the empirical formula i e.

The molecular formula tells you which atoms are present in the compound and how many of each are present. Naming binary molecular compounds. Molecular formula or chemical formula of a compound is the representation of the types of atoms and their ratios present in that compound. The molecular formula tells you which atoms are present in the compound and how many of each are present. Molecular formulas give the kind and number of atoms of each element present in the molecular compound.
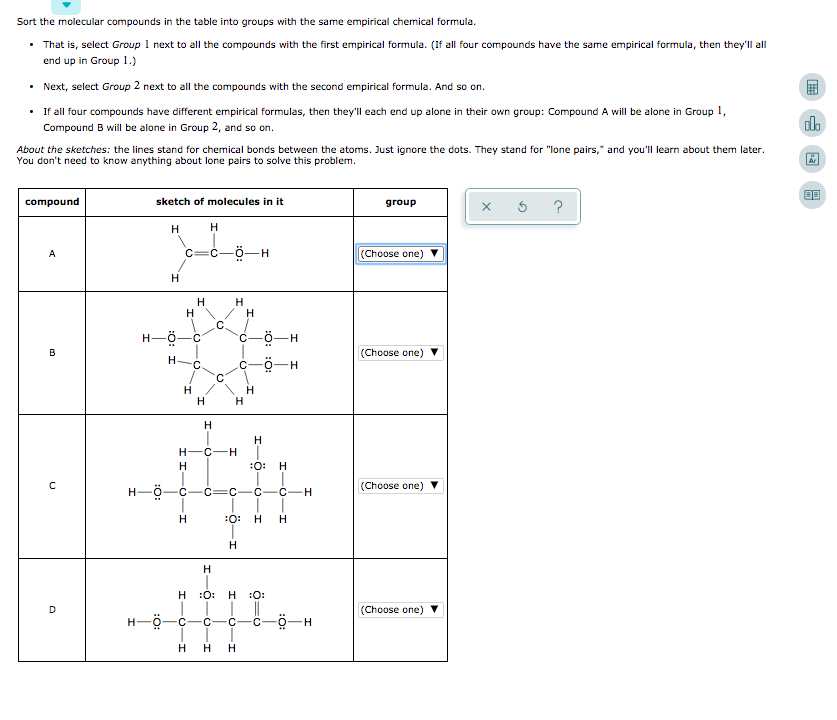 Source: chegg.com
Source: chegg.com
Naming binary molecular compounds. Ch 3 coo 5. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Molecular formulas give the kind and number of atoms of each element present in the molecular compound. Sulfuric acid 98 072 g mol.
 Source: youtube.com
Source: youtube.com
In many cases the molecular formula is the same as the empirical formula. Ch 3 coo 5. H 3 po 4. The formula of a compound which not only expresses the relative number of atoms of each kind but also expresses the actual number of atoms of each element present in one molecule. In molecular formulae the elements are denoted by their respective symbols as in the periodic table and the number of atoms of each element in the molecule is written in subscript.
Source: chem.fsu.edu
H 3 po 4. The molecular formula tells you which atoms are present in the compound and how many of each are present. A molecule of water contains two hydrogen atoms and one oxygen atom so its formula is ce h 2o. Molecular formulas give the kind and number of atoms of each element present in the molecular compound. Empirical formula represents the simplest whole number ratio of all kinds of atoms present in a molecule of a compound.
 Source: wou.edu
Source: wou.edu
Molecular formulas give the kind and number of atoms of each element present in the molecular compound. H 2 so 4. The molecular formula is an important piece of information for any chemical compound. Naming binary molecular compounds. Molecular formula tells the exact number and type of atoms in a single molecule of a compound.
 Source: slideplayer.com
Source: slideplayer.com
A molecule of water contains two hydrogen atoms and one oxygen atom so its formula is ce h 2o. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Naming binary molecular compounds. The molecular formula tells you which atoms are present in the compound and how many of each are present. Na 3 po 4.

Na 3 po 4. In many cases the molecular formula is the same as the empirical formula. H 3 po 4. You will need to know the empirical formula to calculate the molecular formula and you will need to know that the difference between these two formulas is a whole number multiplier. Chemical elements periodic table compound name formula search moles to grams calculator common compounds list chemical equation balancer complete list of acids complete list of bases molar to mass concentration converter molar mass calculator cations anions list dilution calculator molarity calculator.
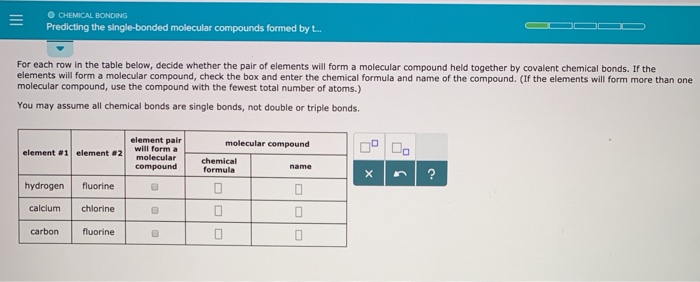 Source: chegg.com
Source: chegg.com
The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Recall that a molecular formula shows the number of atoms of each element that a molecule contains. The molecular formula is an important piece of information for any chemical compound. The molecular formula tells you which atoms are present in the compound and how many of each are present. The formula of a compound which not only expresses the relative number of atoms of each kind but also expresses the actual number of atoms of each element present in one molecule.
 Source: researchgate.net
Source: researchgate.net
Molecular formula or chemical formula of a compound is the representation of the types of atoms and their ratios present in that compound. A molecule of octane which is a component of gasoline contains 8 atoms of carbon and 18 atoms of hydrogen. Sulfuric acid 98 072 g mol. Na 3 po 4. A molecule of water contains two hydrogen atoms and one oxygen atom so its formula is ce h 2o.
 Source: youtube.com
Source: youtube.com
The molecular formula of a compound is defined as. The chemical formula will always be some integer multiple n of the empirical formula i e. Molecular formula or chemical formula of a compound is the representation of the types of atoms and their ratios present in that compound. In molecular formulae the elements are denoted by their respective symbols as in the periodic table and the number of atoms of each element in the molecule is written in subscript. H 3 po 4.
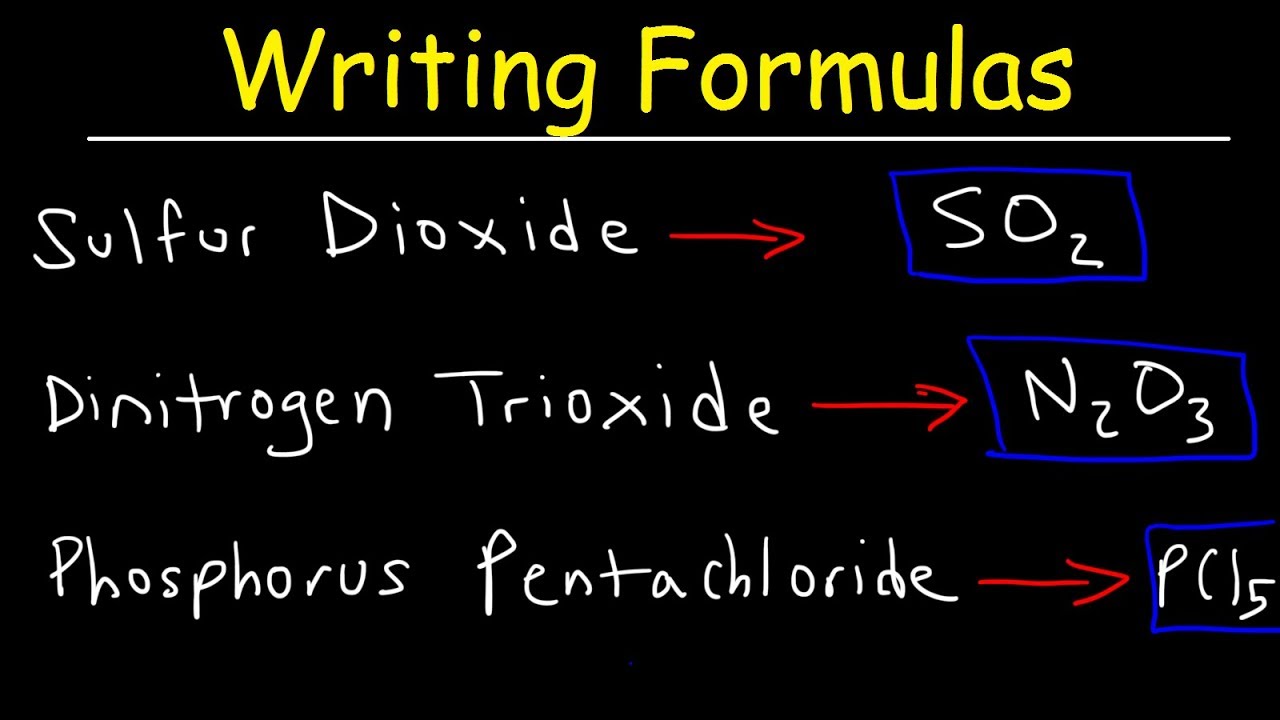 Source: youtube.com
Source: youtube.com
You will need to know the empirical formula to calculate the molecular formula and you will need to know that the difference between these two formulas is a whole number multiplier. Ch 3 coo 5. You will need to know the empirical formula to calculate the molecular formula and you will need to know that the difference between these two formulas is a whole number multiplier. The molecular formula is an important piece of information for any chemical compound. The molecular formula provides insight into the number of elements present in a compound.
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
Naming binary molecular compounds. Chemical elements periodic table compound name formula search moles to grams calculator common compounds list chemical equation balancer complete list of acids complete list of bases molar to mass concentration converter molar mass calculator cations anions list dilution calculator molarity calculator. The molecular formula provides insight into the number of elements present in a compound. You will need to know the empirical formula to calculate the molecular formula and you will need to know that the difference between these two formulas is a whole number multiplier. The molecular formula is an important piece of information for any chemical compound.
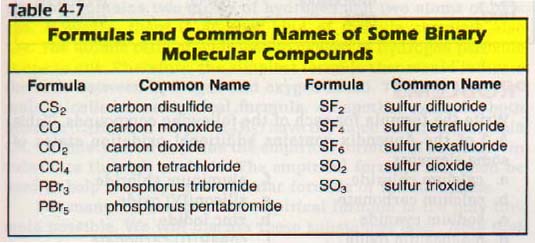 Source: boomeria.org
Source: boomeria.org
Naming binary molecular compounds. The molecular formula for a compound can be the same as or a multiple of the compound s empirical formula. The formula of a compound which not only expresses the relative number of atoms of each kind but also expresses the actual number of atoms of each element present in one molecule. Chemical elements periodic table compound name formula search moles to grams calculator common compounds list chemical equation balancer complete list of acids complete list of bases molar to mass concentration converter molar mass calculator cations anions list dilution calculator molarity calculator. The molecular formula of a compound is defined as.
 Source: youtube.com
Source: youtube.com
Ch 3 coo 5. Molecular formula tells the exact number and type of atoms in a single molecule of a compound. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. H 2 so 4. For example the molecular formula for glucose is c 6 h 12 o 6.
 Source: slideplayer.com
Source: slideplayer.com
A molecule of octane which is a component of gasoline contains 8 atoms of carbon and 18 atoms of hydrogen. A molecule of octane which is a component of gasoline contains 8 atoms of carbon and 18 atoms of hydrogen. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Molecular formula or chemical formula of a compound is the representation of the types of atoms and their ratios present in that compound. Naming binary molecular compounds.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular compound formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.