Most reactive metal
Most Reactive Metal. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. The most reactive metal in the periodic table is francium. However the quantity of francium produced until now is too little. It exists in gaseous form even at room temperature.
 Activity Of Metals From chemed.chem.purdue.edu
Activity Of Metals From chemed.chem.purdue.edu
A generally liquids c generally solids and gases b generally gases d generally gases and liquids 4. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. The liquid metal at room temperature a mercury c sodium b bromine d gold 3. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Francium is the most reactive metal on the periodic table. Therefore for practical purposes cesium is often regarded as the most reactive metal.
Therefore for practical purposes cesium is often regarded as the most reactive metal.
The most reactive metal is a iron b zinc c gold d potassium 2. Cesium and francium are the most reactive metals and are at the top of the reactivity series. The most reactive metal in the periodic table is francium. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. The metal which is stored in kerosene.
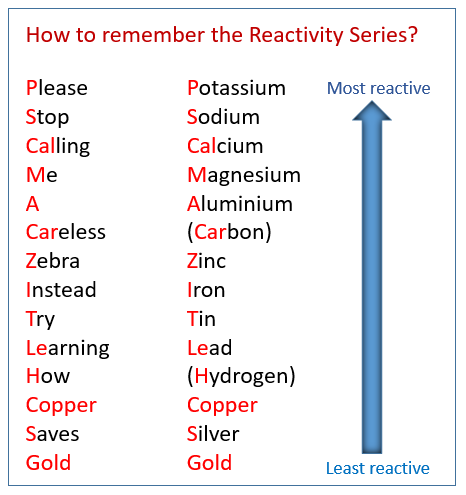 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The most reactive metal is a iron b zinc c gold d potassium 2. The most reactive metal in the periodic table is francium. However the quantity of francium produced until now is too little. Francium is the most reactive metal on the periodic table. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. It is almost impossible to find it in pure form. A generally liquids c generally solids and gases b generally gases d generally gases and liquids 4. The metal is also radioactive and one of the rarest naturally occurring elements. The most reactive metal in the periodic table is francium.
Source: quora.com
This is due to its highest electronegativity. The metal which is stored in kerosene. Francium is the most reactive metal on the periodic table. The most reactive metal on the periodic table is francium. However the quantity of francium produced until now is too little.

Therefore for practical purposes cesium is often regarded as the most reactive metal. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. The most reactive metal on the periodic table is francium. The liquid metal at room temperature a mercury c sodium b bromine d gold 3. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
 Source: kids.britannica.com
Source: kids.britannica.com
The most reactive metal in the periodic table is francium. However the quantity of francium produced until now is too little. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium.
Source: excellup.com
A generally liquids c generally solids and gases b generally gases d generally gases and liquids 4. Therefore for practical purposes cesium is often regarded as the most reactive metal. The metal which is stored in kerosene. These metals are highly reactive because they all have only one valence electron. The most reactive metal on the periodic table is francium.
 Source: pinterest.co.uk
Source: pinterest.co.uk
Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. The metal is also radioactive and one of the rarest naturally occurring elements. It is almost impossible to find it in pure form. It even reacts with glass.
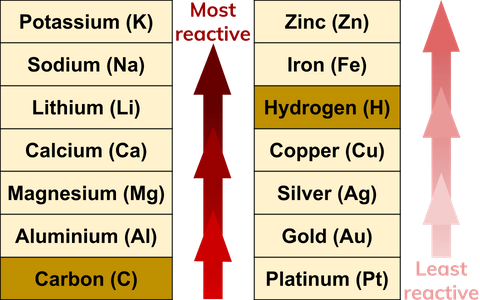 Source: senecalearning.com
Source: senecalearning.com
Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. In the periodic table fluorine is the most reactive element. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. This is due to its highest electronegativity. However the quantity of francium produced until now is too little.
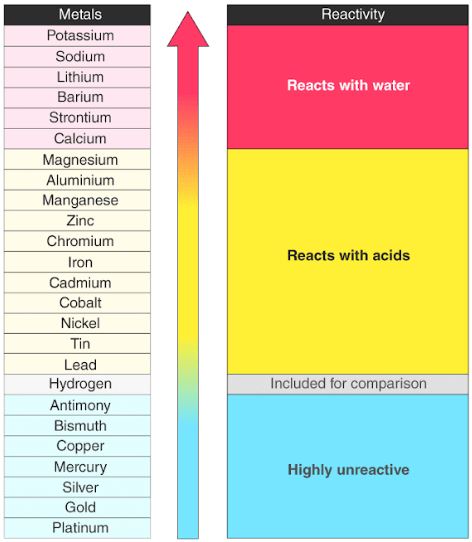 Source: byjus.com
Source: byjus.com
Therefore for practical purposes cesium is often regarded as the most reactive metal. These metals are highly reactive because they all have only one valence electron. The most reactive metal in the periodic table is francium. Cesium and francium are the most reactive metals and are at the top of the reactivity series. The metal is also radioactive and one of the rarest naturally occurring elements.
 Source: youtube.com
Source: youtube.com
The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. These metals are highly reactive because they all have only one valence electron. In the periodic table fluorine is the most reactive element. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive.
 Source: compoundchem.com
Source: compoundchem.com
It exists in gaseous form even at room temperature. The most reactive metal in the periodic table is francium. Francium is the most reactive metal on the periodic table. The most reactive metal is a iron b zinc c gold d potassium 2. The metal is also radioactive and one of the rarest naturally occurring elements.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. The metal is also radioactive and one of the rarest naturally occurring elements. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. However the quantity of francium produced until now is too little.
 Source: socratic.org
Source: socratic.org
2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Francium is the most reactive metal on the periodic table. The metal which is stored in kerosene. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt.
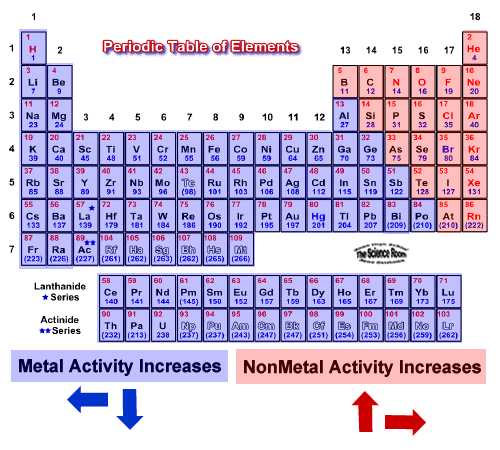 Source: socratic.org
Source: socratic.org
The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. It exists in gaseous form even at room temperature. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. However the quantity of francium produced until now is too little. The most reactive metal on the periodic table is francium.
 Source: toppr.com
Source: toppr.com
The most reactive metal is a iron b zinc c gold d potassium 2. Cesium and francium are the most reactive metals and are at the top of the reactivity series. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. The most reactive metal is a iron b zinc c gold d potassium 2. This is due to its highest electronegativity.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title most reactive metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







