Most reactive metal element
Most Reactive Metal Element. The most reactive and the least reactive metal from the metals na al cu ag. The most reactive metal is francium the last alkali metal and most expensive element. The least reactive elements are the noble gases. The element in group 17 viia of the periodic table which bleaches vegetables dyes.
 Alkali Metals Group 1 Metals Or Li Na K Rb Cs Fr Squeaky Pop Ppt Download From slideplayer.com
Alkali Metals Group 1 Metals Or Li Na K Rb Cs Fr Squeaky Pop Ppt Download From slideplayer.com
Most reactive metal caesium. The most reactive element is fluorine the first element in the halogen group. The most reactive metal is francium the last alkali metal and most expensive element. Caesium resides in the first column and the second to last row. The least reactive elements are the noble gases. Most reactive na least ag34.
As we ve discussed the radius of an element so low in the column is pretty large compared to the elements above.
The period which contains 8 elements including the non metal sulphur. Caesium resides in the first column and the second to last row. Its atomic number is 55. The period which contains 8 elements including the non metal sulphur. The element in group 17 viia of the periodic table which bleaches vegetables dyes. The least reactive elements are the noble gases.
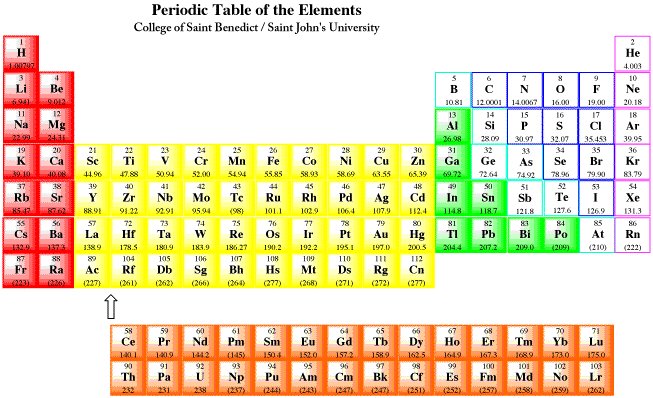 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Caesium is a gold colored metal that reacts explosively with air and water. The metal is also radioactive and one of the rarest naturally occurring elements. The most reactive metal on the periodic table is francium. Most reactive na least ag34. Fluorine is one of the most reactive elements.
 Source: toppr.com
Source: toppr.com
Since beryllium is a highly reactive metal reduction of its compounds requires the use of strong reducing metals. The most reactive metal is francium the last alkali metal and most expensive element. Therefore for practical purposes cesium is often regarded as the most reactive metal. The most reactive and the least reactive metal from the metals na al cu ag 34. The element in group 17 viia of the periodic table which is a liquid at ordinary temperatures 35.
 Source: thoughtco.com
Source: thoughtco.com
The element in group 17 viia of the periodic table which is a liquid at ordinary temperatures 35. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Francium is the most reactive metal on the periodic table. Caesium resides in the first column and the second to last row. Add your answer and earn points.
Source: quora.com
Add your answer and earn points. Therefore for practical purposes cesium is often regarded as the most reactive metal. Most reactive metal caesium. Francium is the most reactive metal on the periodic table. The least reactive elements are the noble gases.
Source: quora.com
The most reactive metal on the periodic table is francium. The most reactive metal on the periodic table is francium. Most reactive na least ag34. Therefore for practical purposes cesium is often regarded as the most reactive metal. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory.
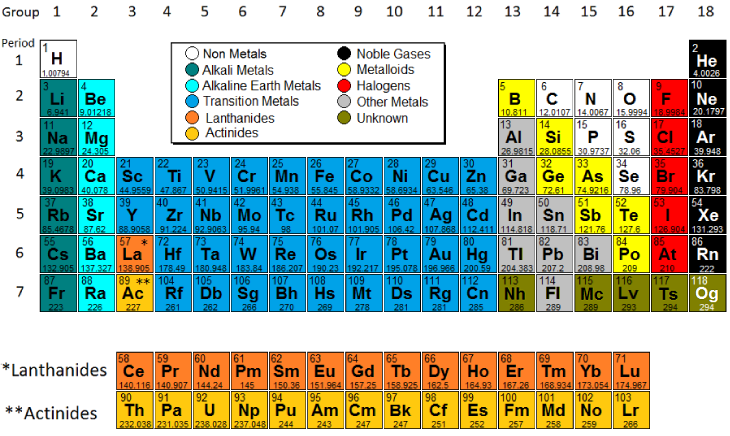 Source: modelscience.com
Source: modelscience.com
Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. The metal is also radioactive and one of the rarest naturally occurring elements. The most reactive and the least reactive metal from the metals na al cu ag. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. The most reactive element is fluorine the first element in the halogen group.
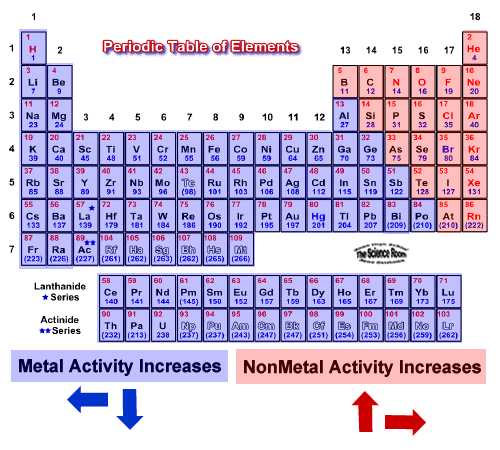 Source: socratic.org
Source: socratic.org
Therefore for practical purposes cesium is often regarded as the most reactive metal. The most reactive metal is francium the last alkali metal and most expensive element. The period which contains 8 elements including the non metal sulphur. Add your answer and earn points. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory.
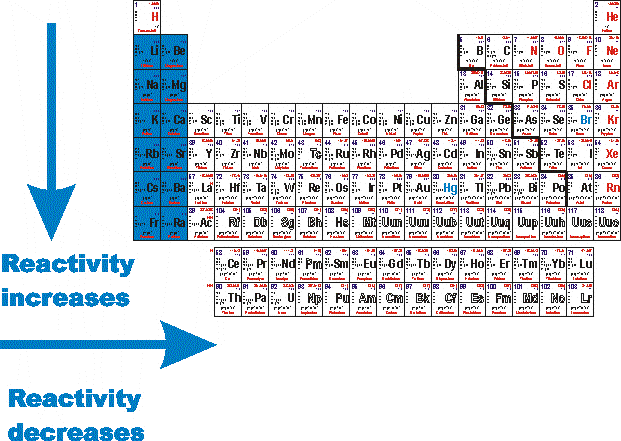 Source: socratic.org
Source: socratic.org
The most reactive element is fluorine the first element in the halogen group. Therefore for practical purposes cesium is often regarded as the most reactive metal. The most reactive and the least reactive metal from the metals na al cu ag. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. The most reactive metal elements is group 1 see answer johnylit is waiting for your help.
Source: quora.com
Most reactive na least ag34. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Reactivity increases as you move down the alkali metals group. The most reactive element is fluorine the first element in the halogen group. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium.
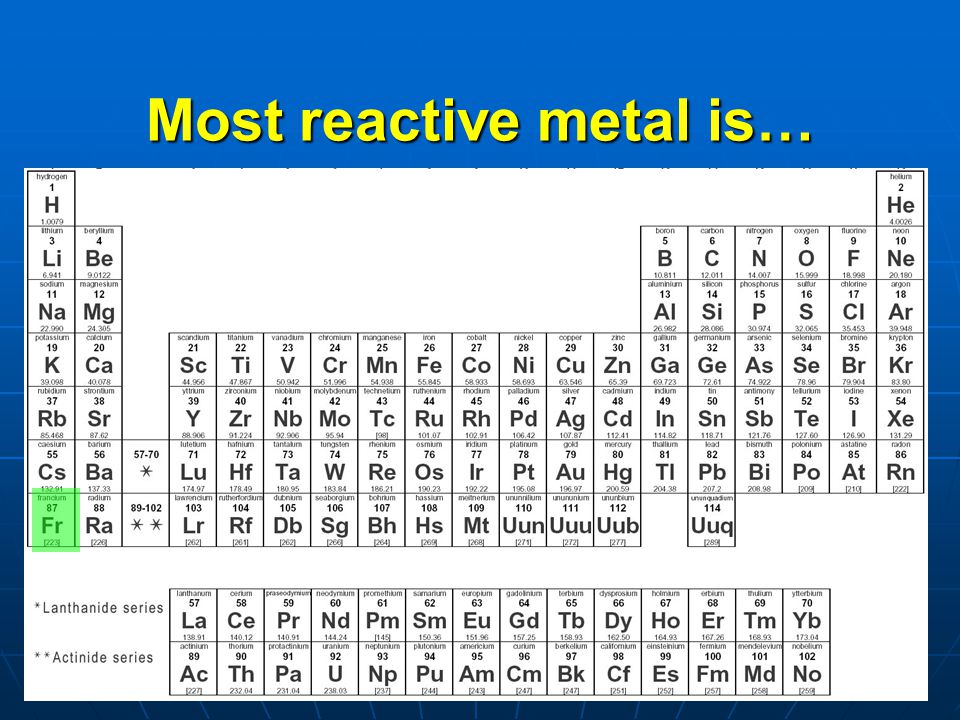 Source: periodictable.me
Source: periodictable.me
The most reactive metals belong to the alkali metals element group. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Francium is the most reactive metal on the periodic table. The least reactive elements are the noble gases. Therefore for practical purposes cesium is often regarded as the most reactive metal.
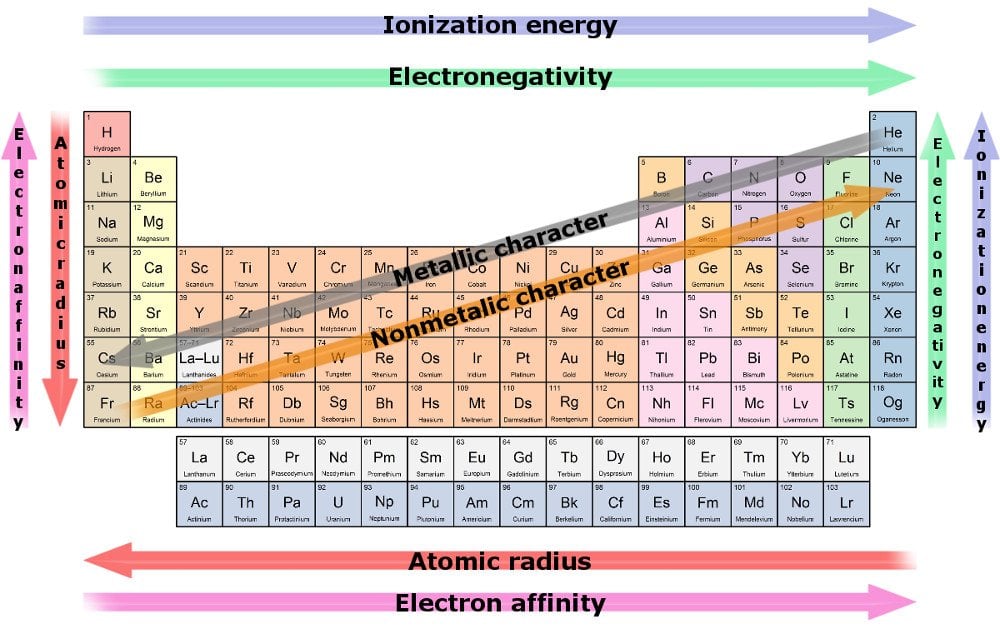 Source: scienceabc.com
Source: scienceabc.com
In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. The most reactive metal elements is group 1 see answer johnylit is waiting for your help. Since beryllium is a highly reactive metal reduction of its compounds requires the use of strong reducing metals. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Most reactive na least ag34. Add your answer and earn points. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. The element in brainly in 33. The most reactive metal elements is group 1 see answer johnylit is waiting for your help.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Since beryllium is a highly reactive metal reduction of its compounds requires the use of strong reducing metals. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Caesium resides in the first column and the second to last row. The most reactive element is fluorine the first element in the halogen group. The most reactive metal on the periodic table is francium.
 Source: slideplayer.com
Source: slideplayer.com
Its atomic number is 55. The most reactive metals belong to the alkali metals element group. Therefore for practical purposes cesium is often regarded as the most reactive metal. Most reactive na least ag34. As we ve discussed the radius of an element so low in the column is pretty large compared to the elements above.
 Source: compoundchem.com
Source: compoundchem.com
Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. The element in group 17 viia of the periodic table which bleaches vegetables dyes. Reactivity increases as you move down the alkali metals group.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title most reactive metal element by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.