Nitrogen oxygen compounds
Nitrogen Oxygen Compounds. Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms. Verify the law of multiple proportions. Nci thesaurus ncit contents.
 Trinitramide Is A Compound Of Nitrogen And Oxygen It Is One Stock Photo Picture And Royalty Free Image Image 50651312 From 123rf.com
Trinitramide Is A Compound Of Nitrogen And Oxygen It Is One Stock Photo Picture And Royalty Free Image Image 50651312 From 123rf.com
Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. Recall that a nitrogen atom has a valence of 3 and has five valence electrons. From wikipedia the free encyclopedia. Most azides are unstable and highly sensitive to shock. In this way the nitrogen present is converted to ammonium sulfate. Nci thesaurus ncit contents.
Verify the law of multiple proportions.
Nci thesaurus ncit contents. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms. Nitrogen forms five compounds with oxygen in which 1 0 g of nitrogen combines with 0 572 1 14 1 73 2 28 and 2 85 of oxygen respectively. 1 structures expand this section. Air is a mixture of gases making up the earth s atmosphere consisting mainly of nitrogen oxygen argon and carbon dioxide. In this way the nitrogen present is converted to ammonium sulfate.
 Source: meritnation.com
Source: meritnation.com
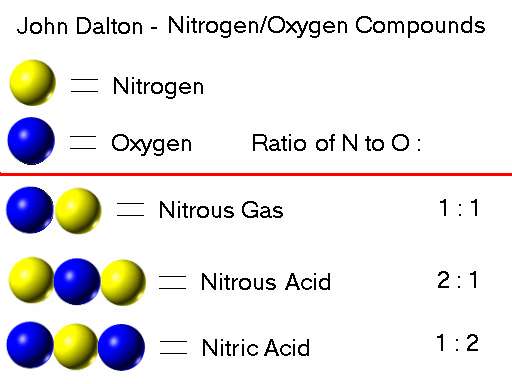
In this way the nitrogen present is converted to ammonium sulfate. Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. Cid 977 oxygen cid 947 nitrogen dates. Nitrogen dioxide contains 2 28 g oxygen to every 1 00 g nitrogen while dinitrogen monoxide. Verify the law of multiple proportions.
 Source: physicsconcepts.org
Source: physicsconcepts.org
Most azides are unstable and highly sensitive to shock. Many compounds composed primarily of carbon and hydrogen also contain some oxygen or nitrogen or one or more of the halogens. Some of them such as lead azide pb n 3 2 are used in detonators and percussion caps. Azides which may be either inorganic or organic are compounds that contain three nitrogen atoms as a group represented as n 3. The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon and hydrogen.
 Source: researchgate.net
Source: researchgate.net
Many compounds composed primarily of carbon and hydrogen also contain some oxygen or nitrogen or one or more of the halogens. Some of them such as lead azide pb n 3 2 are used in detonators and percussion caps. Nitrogen forms several compounds with oxygen including nitrogen dioxide and dinitrogen monoxide. In this way the nitrogen present is converted to ammonium sulfate. Many compounds composed primarily of carbon and hydrogen also contain some oxygen or nitrogen or one or more of the halogens.
 Source: britannica.com
Source: britannica.com
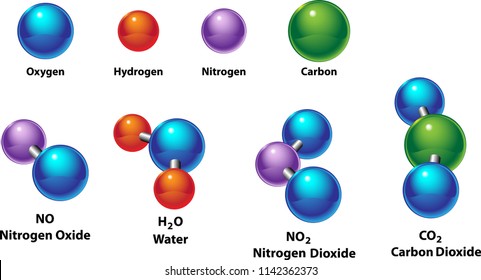
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen or a mixture of such compounds. Many compounds composed primarily of carbon and hydrogen also contain some oxygen or nitrogen or one or more of the halogens. This list may not reflect recent changes. Recall that a nitrogen atom has a valence of 3 and has five valence electrons. Most azides are unstable and highly sensitive to shock.
 Source: researchgate.net
Source: researchgate.net
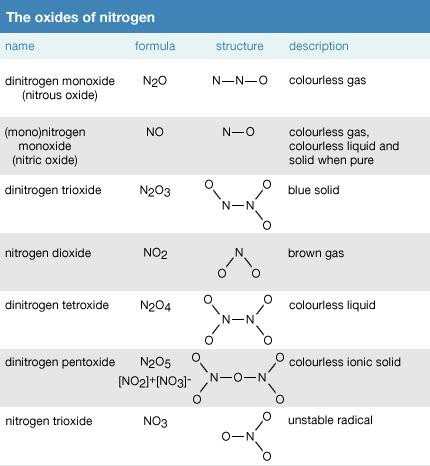
Nitrous oxide n 2 o nitrogen 0 ii oxide. This list may not reflect recent changes. Nitrogen forms five compounds with oxygen in which 1 0 g of nitrogen combines with 0 572 1 14 1 73 2 28 and 2 85 of oxygen respectively. The well known kjeldahl method for determining the nitrogen content of organic compounds involves digestion of the compound with concentrated sulfuric acid optionally containing mercury or its oxide and various salts depending on the nature of the nitrogen compound. Some of them such as lead azide pb n 3 2 are used in detonators and percussion caps.
Source: chem.libretexts.org
The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon and hydrogen. Most azides are unstable and highly sensitive to shock. Verify the law of multiple proportions. Azides which may be either inorganic or organic are compounds that contain three nitrogen atoms as a group represented as n 3. Nitrous oxide n 2 o nitrogen 0 ii oxide.
 Source: shutterstock.com
Source: shutterstock.com
Nitrous oxide n 2 o nitrogen 0 ii oxide. Recall that a nitrogen atom has a valence of 3 and has five valence electrons. Verify the law of multiple proportions. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms. This list may not reflect recent changes.
 Source: 123rf.com
Source: 123rf.com
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen or a mixture of such compounds. Nitrous oxide n 2 o nitrogen 0 ii oxide. Verify the law of multiple proportions. 1 structures expand this section. Many compounds composed primarily of carbon and hydrogen also contain some oxygen or nitrogen or one or more of the halogens.
 Source: researchgate.net
Source: researchgate.net
Nitrogen occurs in all organisms primarily in amino acids and thus proteins in the nucleic acids dna and rna and in the energy transfer molecule adenosine triphosphate. Nitrogen occurs in all organisms primarily in amino acids and thus proteins in the nucleic acids dna and rna and in the energy transfer molecule adenosine triphosphate. Pages in category nitrogen oxygen compounds the following 11 pages are in this category out of 11 total. Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. Some of them such as lead azide pb n 3 2 are used in detonators and percussion caps.

We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms. Nitrogen oxide may refer to a binary compound of oxygen and nitrogen or a mixture of such compounds. This list may not reflect recent changes. Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. Most azides are unstable and highly sensitive to shock.
 Source: askiitians.com
Source: askiitians.com
Azides which may be either inorganic or organic are compounds that contain three nitrogen atoms as a group represented as n 3. 1 structures expand this section. In this way the nitrogen present is converted to ammonium sulfate. From wikipedia the free encyclopedia. Nitrogen occurs in all organisms primarily in amino acids and thus proteins in the nucleic acids dna and rna and in the energy transfer molecule adenosine triphosphate.
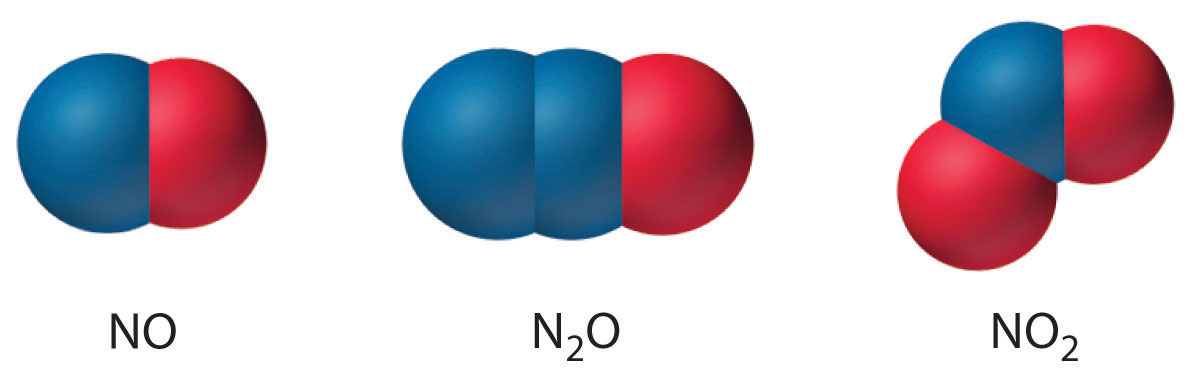 Source: chem.libretexts.org
Source: chem.libretexts.org
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen or a mixture of such compounds. Pages in category nitrogen oxygen compounds the following 11 pages are in this category out of 11 total. In this way the nitrogen present is converted to ammonium sulfate. Verify the law of multiple proportions. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms.
 Source: scied.ucar.edu
Source: scied.ucar.edu
Dinitrogen dioxide n 2 o 2 nitrogen ii oxide dimer. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms. Nitrogen forms several compounds with oxygen including nitrogen dioxide and dinitrogen monoxide. Nitrogen oxide may refer to a binary compound of oxygen and nitrogen or a mixture of such compounds. The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon and hydrogen.
 Source: 123rf.com
Source: 123rf.com
Air is a mixture of gases making up the earth s atmosphere consisting mainly of nitrogen oxygen argon and carbon dioxide. Nitrogen dioxide contains 2 28 g oxygen to every 1 00 g nitrogen while dinitrogen monoxide. The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon and hydrogen. Recall that a nitrogen atom has a valence of 3 and has five valence electrons. Cid 977 oxygen cid 947 nitrogen dates.
 Source: youtube.com
Source: youtube.com
The well known kjeldahl method for determining the nitrogen content of organic compounds involves digestion of the compound with concentrated sulfuric acid optionally containing mercury or its oxide and various salts depending on the nature of the nitrogen compound. 1 structures expand this section. This list may not reflect recent changes. Nitrogen forms several compounds with oxygen including nitrogen dioxide and dinitrogen monoxide. We thus seek to extend our understanding of bonding and stability by developing lewis structures involving these atoms.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nitrogen oxygen compounds by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.