Oxygen compound formula
Oxygen Compound Formula. Boron suboxide chemical formula b 6 o is a solid compound containing six boron atoms and one oxygen atom. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. This molecule is the major naturally existing form of elemental oxygen and is also called dioxygen diatomic oxygen oxygen gas and molecular oxygen. Corrosive to skin and eyes.
 Ch 4 From boomeria.org
Ch 4 From boomeria.org
The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. The molar mass of o 16. 1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. The other natural form of elemental oxygen is o 3 an unstable molecule known as ozone. Molar rate of s.
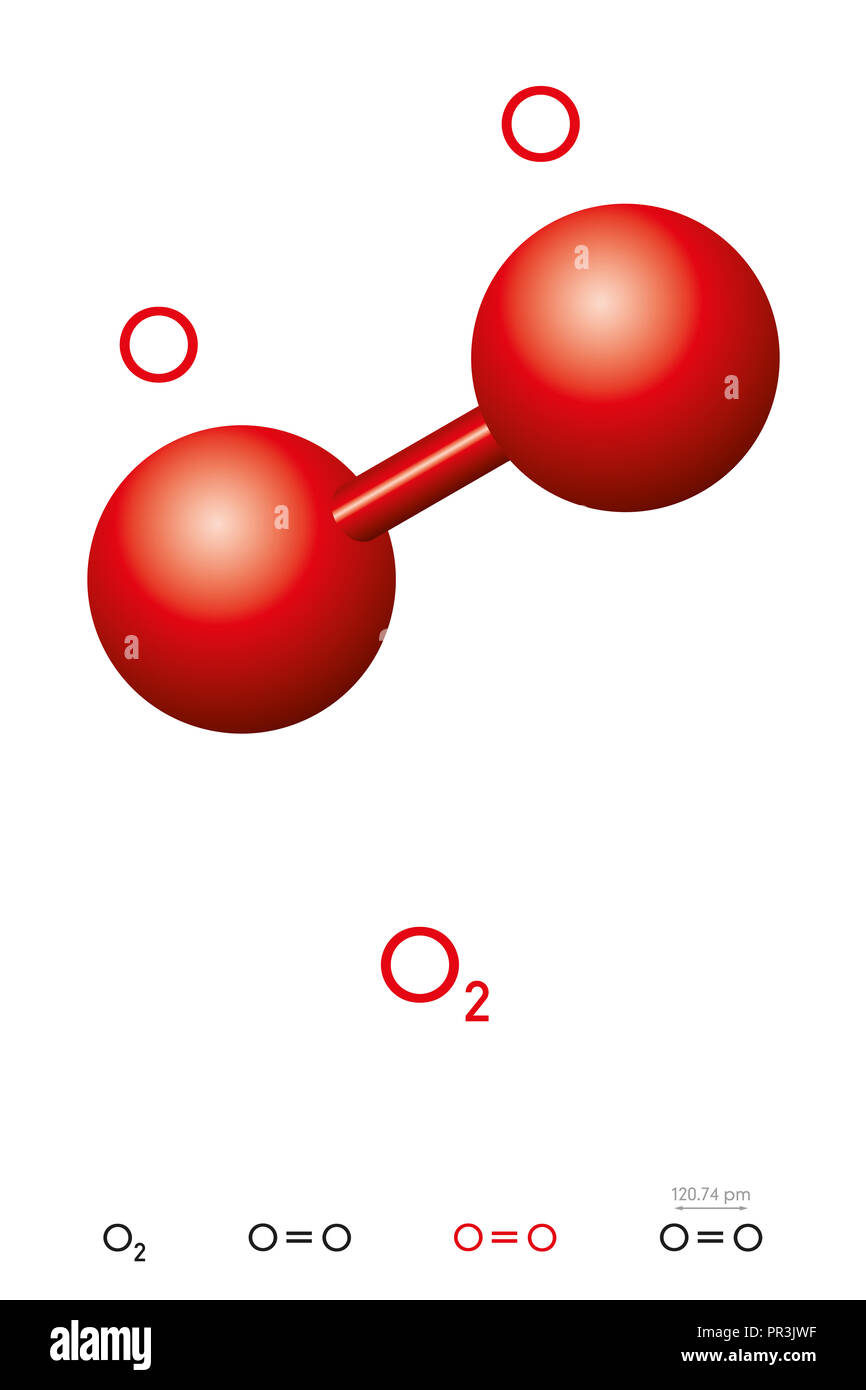
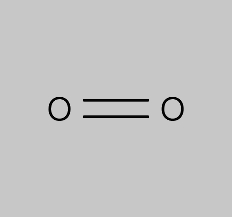
The molecule is diatomic meaning that is formed by two oxygen atoms that are bound through a double bond.
What is the formula of the compound formed between oxygen o and sodium na. Na2o because oxygen gains two electrons and sodium loses one electron. Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature. The oxygen chemical formula is o 2. The number of moles of o 40 16 2 5. As compound it is a gas and the main component of the air being indispensable to keeping the organisms alive.
 Source: britannica.com
Source: britannica.com
Oxygen o2 2 or o2 cid 977 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. The number of moles of o 40 16 2 5. The other natural form of elemental oxygen is o 3 an unstable molecule known as ozone. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. Corrosive to skin and eyes.
 Source: molinstincts.com
Source: molinstincts.com
1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride. Compounds containing oxygen in other oxidation states are very uncommon. Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature. Molar rate of s. 1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride.
 Source: boomeria.org
Source: boomeria.org
The molar mass is 32 00 g mol. The compound has the empirical formula ch 2 o. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. Ona because oxygen gains one electron and sodium loses one electron. The number of moles of o 40 16 2 5.
 Source: youtube.com
Source: youtube.com
Nao because oxygen gains one electron and sodium. As compound it is a gas and the main component of the air being indispensable to keeping the organisms alive. The molar mass of o 16. Highly toxic by inhalation. Boron suboxide chemical formula b 6 o is a solid compound containing six boron atoms and one oxygen atom.
 Source: quora.com
Source: quora.com
Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. The number of moles of o 40 16 2 5. As compound it is a gas and the main component of the air being indispensable to keeping the organisms alive. Boron suboxide chemical formula b 6 o is a solid compound containing six boron atoms and one oxygen atom.
 Source: mikeblaber.org
Source: mikeblaber.org
The empirical formula would be so2. This molecule is the major naturally existing form of elemental oxygen and is also called dioxygen diatomic oxygen oxygen gas and molecular oxygen. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. The compound has the empirical formula ch 2 o. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula.
 Source: evolvingsciences.com
Source: evolvingsciences.com
Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Na2o because oxygen gains two electrons and sodium loses one electron. 1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride. The empirical formula would be so2. Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature.
 Source: alamy.com
Source: alamy.com
The molecule is diatomic meaning that is formed by two oxygen atoms that are bound through a double bond. Oxygen o2 2 or o2 cid 977 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. What is the formula of the compound formed between oxygen o and sodium na. 1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride. The most common formula for elemental oxygen is o2.
Source: chemspider.com
Boron suboxide chemical formula b 6 o is a solid compound containing six boron atoms and one oxygen atom. As compound it is a gas and the main component of the air being indispensable to keeping the organisms alive. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. The oxygen chemical formula is o 2. Compounds containing oxygen in other oxidation states are very uncommon.
 Source: 123rf.com
Source: 123rf.com
Compounds containing oxygen in other oxidation states are very uncommon. The compound has the empirical formula ch 2 o. This molecule is the major naturally existing form of elemental oxygen and is also called dioxygen diatomic oxygen oxygen gas and molecular oxygen. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. Compounds containing oxygen in other oxidation states are very uncommon.
 Source: byjus.com
Source: byjus.com
The other natural form of elemental oxygen is o 3 an unstable molecule known as ozone. Highly toxic by inhalation. The empirical formula would be so2. This molecule is the major naturally existing form of elemental oxygen and is also called dioxygen diatomic oxygen oxygen gas and molecular oxygen. The compound has the empirical formula ch 2 o.
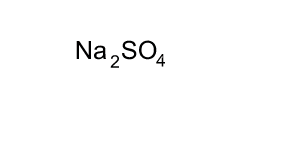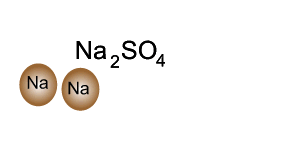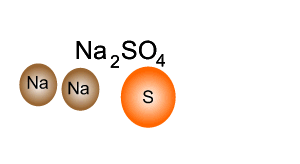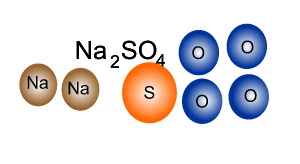 Source: dynamicscience.com.au
Source: dynamicscience.com.au
1 2 superoxides 1 3 0 elemental hypofluorous acid 1 2 1 dioxygen difluoride and 2 oxygen difluoride. Nao because oxygen gains one electron and sodium. This molecule is the major naturally existing form of elemental oxygen and is also called dioxygen diatomic oxygen oxygen gas and molecular oxygen. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. Corrosive to skin and eyes.
 Source: study.com
Source: study.com
Ona because oxygen gains one electron and sodium loses one electron. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. The molecule is diatomic meaning that is formed by two oxygen atoms that are bound through a double bond. The empirical formula would be so2.
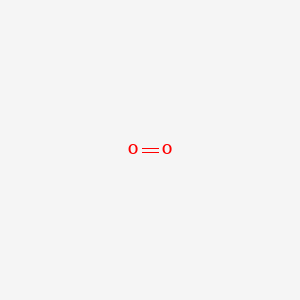
Ona2 because oxygen gains two electrons and sodium loses one electron. Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature. The actual number of atoms within each particle of the compound is some multiple of the numbers expressed in this formula. Corrosive to skin and eyes. The molecule is diatomic meaning that is formed by two oxygen atoms that are bound through a double bond.
 Source: nde-ed.org
Source: nde-ed.org
The oxygen chemical formula is o 2. The number of moles of o 40 16 2 5. The compound has the empirical formula ch 2 o. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. Can explode on contact with water decomposes to toxic gaseous fluorine if heated to high temperature.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title oxygen compound formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.