Phenolphthalein colour change
Phenolphthalein Colour Change. Between strongly acidic and slightly basic conditions the lactone form hin is colorless. Phenolphthalein adopts at least four different states in aqueous solution as a result of ph changes. The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules. Also it turns in pink colour in an alkaline solution or base.
 Acid Base Indicators From chemguide.co.uk
Acid Base Indicators From chemguide.co.uk
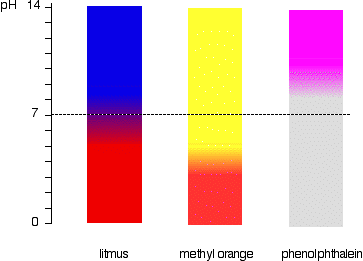
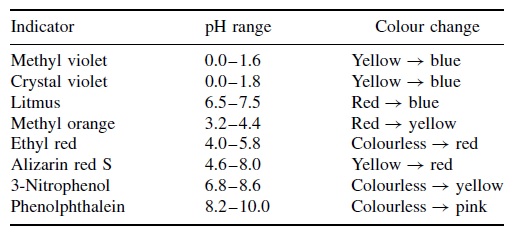
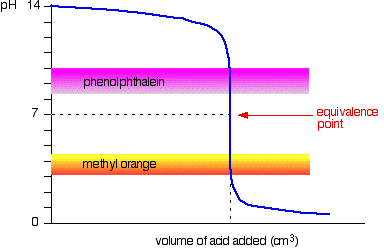
The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself. Phenolphthalein changes colour at a ph above 7. It so happens that the phenolphthalein has finished its colour change at exactly the ph of the equivalence point of the first half of the reaction in which sodium hydrogencarbonate is produced. Colour change of phenolphthalein. Moreover the compound remains colourless in acids but starts to turn pink on ph 8 2 and continues to turn bright purple in strong bases. The natural colour of phenolphthalein is colourless.
In acidic solutions it is colorless.
Also it turns in pink colour in an alkaline solution or base. In acid solution phenolphthalein indicator gives colourless. Under strongly acidic conditions it exists in protonated form hin providing an orange coloration. Phenolphthalein is naturally colourless and works differently then litmus paper. Phenolphthalein c 20 h 14 o 4 an organic compound of the phthalein family that is widely employed as an acid base indicator. It is also suitable for titrations of weak acids and strong bases which have an equivalence point at a ph above 7 methyl orange changes colour at a suitable ph.
 Source: socratic.org
Source: socratic.org
As an indicator of a solution s ph phenolphthalein is colourless below ph 8 5 and attains a pink to deep red hue above ph 9 0. Phenolphthalein adopts at least four different states in aqueous solution as a result of ph changes. It is also suitable for titrations of weak acids and strong bases which have an equivalence point at a ph above 7 methyl orange changes colour at a suitable ph. Recall that ph indicators are not only natural dyes but also weak acids. As an indicator of a solution s ph phenolphthalein is colourless below ph 8 5 and attains a pink to deep red hue above ph 9 0.
 Source: compoundchem.com
Source: compoundchem.com
In acid solution phenolphthalein indicator gives colourless. Moreover the compound remains colourless in acids but starts to turn pink on ph 8 2 and continues to turn bright purple in strong bases. Also it turns in pink colour in an alkaline solution or base. In acidic solutions it is colorless. Recall that ph indicators are not only natural dyes but also weak acids.
 Source: researchgate.net
Source: researchgate.net
In acid solution phenolphthalein indicator gives colourless. Stir the water to evenly distribute the pink color indicator. The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself. Phenolphthalein adopts at least four different states in aqueous solution as a result of ph changes. The natural colour of phenolphthalein is colourless.

Also it turns in pink colour in an alkaline solution or base. Also it turns in pink colour in an alkaline solution or base. As an indicator of a solution s ph phenolphthalein is colourless below ph 8 5 and attains a pink to deep red hue above ph 9 0. Stir the water to evenly distribute the pink color indicator. Recall that ph indicators are not only natural dyes but also weak acids.
 Source: quora.com
Source: quora.com
Between strongly acidic and slightly basic conditions the lactone form hin is colorless. The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules. It will look like you are pouring pink liquid into the water. Phenolphthalein adopts at least four different states in aqueous solution as a result of ph changes. It so happens that the phenolphthalein has finished its colour change at exactly the ph of the equivalence point of the first half of the reaction in which sodium hydrogencarbonate is produced.
 Source: quora.com
Source: quora.com
It so happens that the phenolphthalein has finished its colour change at exactly the ph of the equivalence point of the first half of the reaction in which sodium hydrogencarbonate is produced. The water will turn shocking pink as the phenolphthalein solution enters. Also it turns in pink colour in an alkaline solution or base. The methyl orange changes colour at exactly the ph of the equivalence point of the second stage of the reaction. The dissociation of the weak acid indicator causes the solution to change color.
 Source: sciencephoto.com
Source: sciencephoto.com
Under strongly acidic conditions it exists in protonated form hin providing an orange coloration. The methyl orange changes colour at exactly the ph of the equivalence point of the second stage of the reaction. It so happens that the phenolphthalein has finished its colour change at exactly the ph of the equivalence point of the first half of the reaction in which sodium hydrogencarbonate is produced. The water will turn shocking pink as the phenolphthalein solution enters. The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules.
 Source: chemguide.co.uk
Source: chemguide.co.uk
So it is quite good as an indicator for titrations of strong acids with strong bases. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. The water will turn shocking pink as the phenolphthalein solution enters. Stir the water to evenly distribute the pink color indicator. Colour change of phenolphthalein.
 Source: chemicalbook.com
Source: chemicalbook.com
It is also suitable for titrations of weak acids and strong bases which have an equivalence point at a ph above 7 methyl orange changes colour at a suitable ph. The natural colour of phenolphthalein is colourless. Admire the brilliant pink. It so happens that the phenolphthalein has finished its colour change at exactly the ph of the equivalence point of the first half of the reaction in which sodium hydrogencarbonate is produced. In acid solution phenolphthalein indicator gives colourless.
 Source: quora.com
Source: quora.com
The natural colour of phenolphthalein is colourless. The methyl orange changes colour at exactly the ph of the equivalence point of the second stage of the reaction. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself. Colour change of phenolphthalein.
 Source: researchgate.net
Source: researchgate.net
Also it turns in pink colour in an alkaline solution or base. Recall that ph indicators are not only natural dyes but also weak acids. It will look like you are pouring pink liquid into the water. As an indicator of a solution s ph phenolphthalein is colourless below ph 8 5 and attains a pink to deep red hue above ph 9 0. The dissociation of the weak acid indicator causes the solution to change color.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself. Admire the brilliant pink. Recall that ph indicators are not only natural dyes but also weak acids. It is also suitable for titrations of weak acids and strong bases which have an equivalence point at a ph above 7 methyl orange changes colour at a suitable ph. The methyl orange changes colour at exactly the ph of the equivalence point of the second stage of the reaction.

The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself. Under strongly acidic conditions it exists in protonated form hin providing an orange coloration. In a basic solution phenolphthalein indicator gives pink colour. Moreover the compound remains colourless in acids but starts to turn pink on ph 8 2 and continues to turn bright purple in strong bases. Phenolphthalein adopts at least four different states in aqueous solution as a result of ph changes.
 Source: chemguide.co.uk
Source: chemguide.co.uk
The water will turn shocking pink as the phenolphthalein solution enters. Stir the water to evenly distribute the pink color indicator. The methyl orange changes colour at exactly the ph of the equivalence point of the second stage of the reaction. Admire the brilliant pink. Phenolphthalein c 20 h 14 o 4 an organic compound of the phthalein family that is widely employed as an acid base indicator.
 Source: brainly.in
Source: brainly.in
The water will turn shocking pink as the phenolphthalein solution enters. Stir the water to evenly distribute the pink color indicator. As an indicator of a solution s ph phenolphthalein is colourless below ph 8 5 and attains a pink to deep red hue above ph 9 0. Pour the phenolphthalein solution from the vial into the glass container. The color change of a ph indicator is caused by the dissociation of the h ion from the indicator itself.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phenolphthalein colour change by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





