Pure substance or mixture examples
Pure Substance Or Mixture Examples. Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Some of the worksheets below are pure substances and mixtures worksheets learn how to differentiate between a pure substance homogeneous mixture and a heterogeneous mixture identify properties of a pure substance exploring the methods useful for the separation of mixtures including distillation extraction filtration decanting centrifuging sublimation. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. Gold is an element.
 What Are Pure Substances And Mixtures Posters Teaching Resource Teach Starter From teachstarter.com
What Are Pure Substances And Mixtures Posters Teaching Resource Teach Starter From teachstarter.com
An atom is the smallest particle of an element that still has all the properties of the element. Pure metals such as gold silver and platinum are good examples for pure solid substances. Examples of pure substances. Some of the worksheets below are pure substances and mixtures worksheets learn how to differentiate between a pure substance homogeneous mixture and a heterogeneous mixture identify properties of a pure substance exploring the methods useful for the separation of mixtures including distillation extraction filtration decanting centrifuging sublimation. A few of them include gold copper oxygen chlorine diamond etc. Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate.
Examples of pure substances.
A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further. These are often found as solids and can be turned into a molten liquid under high temperatures. Pure metals such as gold silver and platinum are good examples for pure solid substances. A few of them include gold copper oxygen chlorine diamond etc. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further.
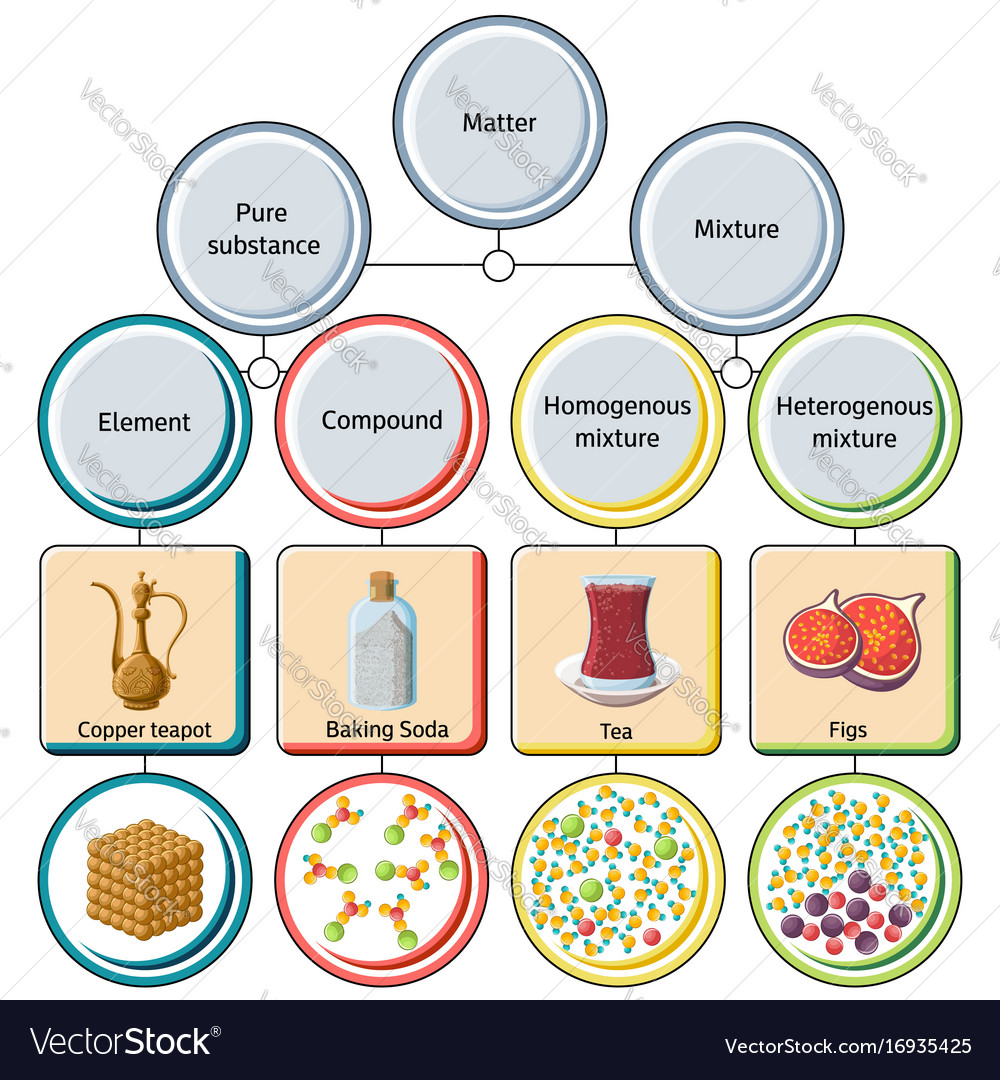 Source: vectorstock.com
Source: vectorstock.com
Ethanol pure grain alcohol the gray area. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. Sugar sucrose baking soda sodium bicarbonate ammonia. Examples of pure substances. A few of them include gold copper oxygen chlorine diamond etc.
 Source: thoughtco.com
Source: thoughtco.com
Examples of pure substances. Sugar sucrose baking soda sodium bicarbonate ammonia. An atom is the smallest particle of an element that still has all the properties of the element. Gold is an element. Pure metals such as gold silver and platinum are good examples for pure solid substances.
 Source: toppr.com
Source: toppr.com
A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. Gold is an element. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary.
 Source: thoughtco.com
Source: thoughtco.com
Some people consider any homogeneous mixture or alloy to be an example of a pure substance. Examples of pure substances include water gases like carbon dioxide oxygen and metals like platinum gold and silver. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. Sugar sucrose baking soda sodium bicarbonate ammonia. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further.
 Source: aplustopper.com
Source: aplustopper.com
Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances. Air is a homogeneous mixture that is often considered to be a pure substance. Examples of pure substances include water gases like carbon dioxide oxygen and metals like platinum gold and silver. Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Some people consider any homogeneous mixture or alloy to be an example of a pure substance.
 Source: bioprofe.com
Source: bioprofe.com
Pure metals such as gold silver and platinum are good examples for pure solid substances. Gold is an element. Air is a homogeneous mixture that is often considered to be a pure substance. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances. Examples of pure substances.
 Source: selftution.com
Source: selftution.com
All elements are mostly pure substances. Air is a homogeneous mixture that is often considered to be a pure substance. An element is composed of a single kind of atom. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances.
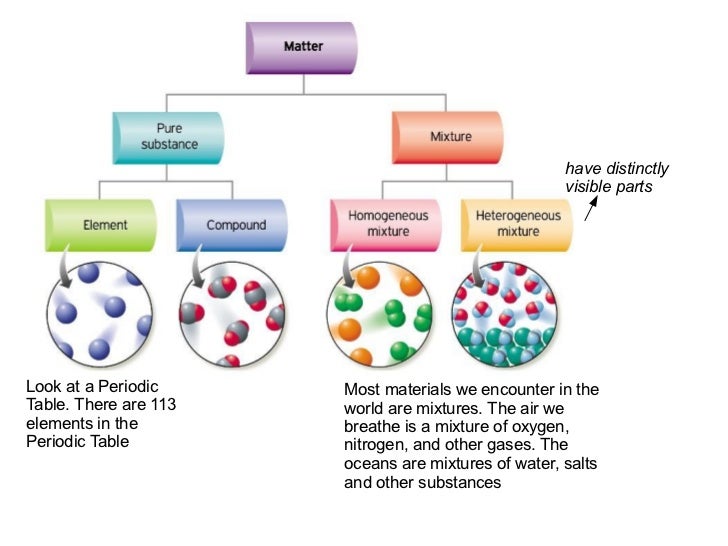 Source: pt.slideshare.net
Source: pt.slideshare.net
Sugar sucrose baking soda sodium bicarbonate ammonia. Sugar sucrose baking soda sodium bicarbonate ammonia. An atom is the smallest particle of an element that still has all the properties of the element. Air is a homogeneous mixture that is often considered to be a pure substance. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further.
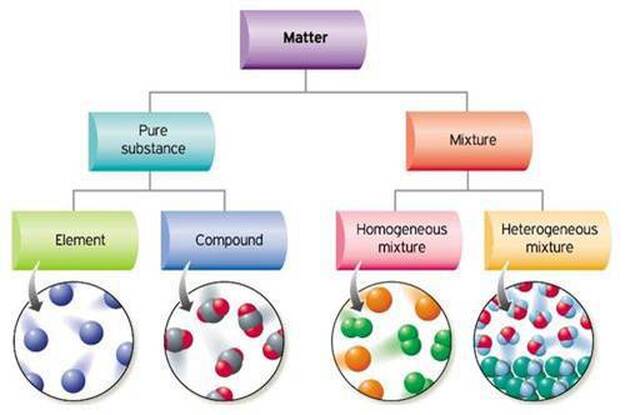 Source: sciencesfp.com
Source: sciencesfp.com
An element is composed of a single kind of atom. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. Some people consider any homogeneous mixture or alloy to be an example of a pure substance. Gold is an element. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Sugar sucrose baking soda sodium bicarbonate ammonia. Pure metals such as gold silver and platinum are good examples for pure solid substances. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances. Examples of pure substances.
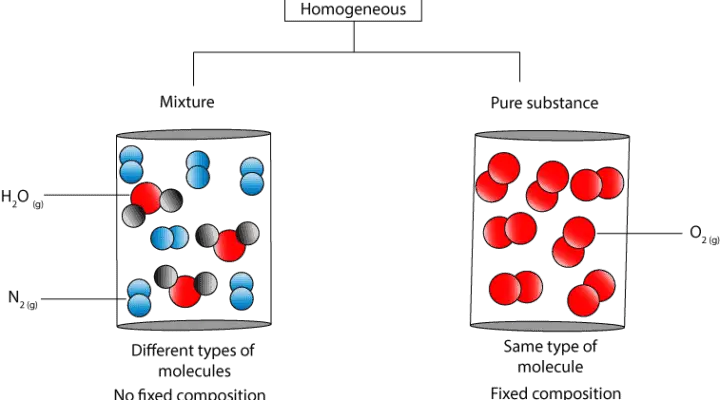 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Examples of pure substances. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances. A few of them include gold copper oxygen chlorine diamond etc. The best examples of pure substances are pure elements molecules and compounds.
 Source: flexiprep.com
Source: flexiprep.com
Examples of pure substances include water gases like carbon dioxide oxygen and metals like platinum gold and silver. Gold is an element. Ethanol pure grain alcohol the gray area. A few of them include gold copper oxygen chlorine diamond etc. Pure metals such as gold silver and platinum are good examples for pure solid substances.
 Source: sciencenotes.org
Source: sciencenotes.org
A few of them include gold copper oxygen chlorine diamond etc. Pure substances have fixed melting points and boiling points and this is very helpful in chemical synthesis to identify unknown substances. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. Examples of pure substances. All elements are mostly pure substances.
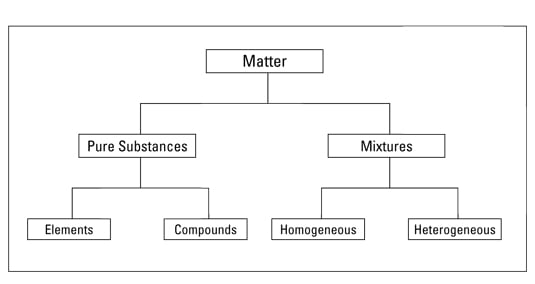 Source: dummies.com
Source: dummies.com
Ethanol pure grain alcohol the gray area. A pure substance can be either an element or a compound but the composition of a pure substance doesn t vary. All elements are mostly pure substances. Ethanol pure grain alcohol the gray area. Some of the worksheets below are pure substances and mixtures worksheets learn how to differentiate between a pure substance homogeneous mixture and a heterogeneous mixture identify properties of a pure substance exploring the methods useful for the separation of mixtures including distillation extraction filtration decanting centrifuging sublimation.
 Source: teachstarter.com
Source: teachstarter.com
Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Examples of pure substances examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Gold is an element. Examples of pure substances include water gases like carbon dioxide oxygen and metals like platinum gold and silver. A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title pure substance or mixture examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





