Reactivity of helium
Reactivity Of Helium. Reaction of helium with water. Since it s completely full it s not happy with sharing or ceding electrons. Based on this what can you infer about the reactivity of helium and neon. Sun is a chemical element with the symbol he and atomic number 2.
 1 Group 0 From slideshare.net
1 Group 0 From slideshare.net
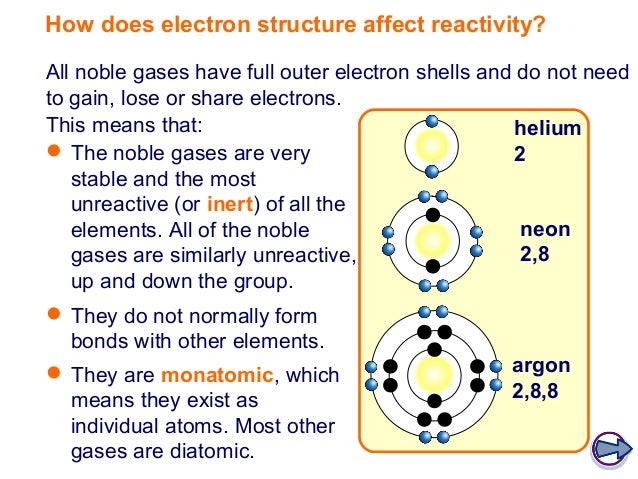
Sometimes referred to as aerogens make up a class of chemical elements with similar properties. Its boiling point is the lowest among all the elements helium is the second lightest and second most abundant element in the observable. It does not react with any other elements or ions so there are no helium bearing minerals in nature. I can infer that helium and neon are not reactive. Helium does not react with any of the halogens. It is a colorless odorless tasteless non toxic inert monatomic gas the first in the noble gas group in the periodic table.
Its boiling point is the lowest among all the elements helium is the second lightest and second most abundant element in the observable.
Its boiling point is the lowest among all the elements helium is the second lightest and second most abundant element in the observable. Well since helium only has one shell it is automatically its outermost shell and it holds 2 electrons which is the most it can have so it is full likewise neon s outermost shell holds 6 out of 6 electrons so it is full so i don t think they would try to form any chemical bonds because they are stable the way they are. Helium is small and extremely light and is the least reactive of all elements. Based on this what can you infer about the reactivity of helium and neon. They re nobile gases meaning they have their outer valence electron shells filled and so practically no reactivity with other atoms. Helium is unreactive because it has a closed shell electron configuration.
 Source: sites.google.com
Source: sites.google.com
Helium was first observed by studying the sun and was named after the greek word for the sun helios. This difference in abundances can be observed in the earth s atmosphere where the ratio of 4 he atoms to 3 he atoms is approximately 1000000 1. Helium does not react with water. It does however dissolve slightly to the extent of about 8 61 cm 3 kg 1 at 20 c 293 k. Helium was first observed by studying the sun and was named after the greek word for the sun helios.
 Source: slideplayer.com
Source: slideplayer.com
How does this row reflect the subshells of the second shell. Its boiling point is the lowest among all the elements helium is the second lightest and second most abundant element in the observable. They re nobile gases meaning they have their outer valence electron shells filled and so practically no reactivity with other atoms. Since it s completely full it s not happy with sharing or ceding electrons. Well since helium only has one shell it is automatically its outermost shell and it holds 2 electrons which is the most it can have so it is full likewise neon s outermost shell holds 6 out of 6 electrons so it is full so i don t think they would try to form any chemical bonds because they are stable the way they are.
 Source: academic.brooklyn.cuny.edu
Source: academic.brooklyn.cuny.edu
Helium has two known stable isotopes 3 he and 4 he. Atoms react and form chemical bonds with other atoms. The atmosphere contains traces of helium. Helium does not react with air even under extreme conditions. Helium is unreactive because it has a closed shell electron configuration.
 Source: slideshare.net
Source: slideshare.net
Since it s completely full it s not happy with sharing or ceding electrons. Helium does not react with any of the halogens. Based on this what can you infer about the reactivity of helium and neon. Under standard conditions they are all odorless colorless monatomic gases with very low chemical reactivity the six naturally occurring noble gases are helium he neon ne argon ar krypton kr xenon xe and the radioactive radon rn. Helium is small and extremely light and is the least reactive of all elements.
 Source: differencebetween.com
Source: differencebetween.com
Under standard conditions they are all odorless colorless monatomic gases with very low chemical reactivity the six naturally occurring noble gases are helium he neon ne argon ar krypton kr xenon xe and the radioactive radon rn. Since it s completely full it s not happy with sharing or ceding electrons. Helium is unreactive because it has a closed shell electron configuration. Under standard conditions they are all odorless colorless monatomic gases with very low chemical reactivity the six naturally occurring noble gases are helium he neon ne argon ar krypton kr xenon xe and the radioactive radon rn. Helium is the least reactive of all of the elements.
 Source: docbrown.info
Source: docbrown.info
Sometimes referred to as aerogens make up a class of chemical elements with similar properties. Helium is small and extremely light and is the least reactive of all elements. Reaction of helium with water. Helium does not react with any of the halogens. The nonreactive properties of helium arise from the fact that it is the lightest of the generally nonreactive noble gases a noble gas has a full electron shell meaning that it cannot easily give or receive electrons in a chemical reaction.
 Source: youtube.com
Source: youtube.com
The abundance of helium 3 and helium 4 corresponds to 0 0002 and 99 9998 respectively. It is a colorless odorless tasteless non toxic inert monatomic gas the first in the noble gas group in the periodic table. The noble gases historically also the inert gases. Helium does not react with air even under extreme conditions. Helium does not react with water.
 Source: slideplayer.com
Source: slideplayer.com
Reaction of helium with air. Reaction of helium with the halogens. The atmosphere contains traces of helium. Helium is small and extremely light and is the least reactive of all elements. Helium was first observed by studying the sun and was named after the greek word for the sun helios.
 Source: quora.com
Source: quora.com
Sun is a chemical element with the symbol he and atomic number 2. Helium does not react with air even under extreme conditions. The noble gases historically also the inert gases. Helium is unreactive because it has a closed shell electron configuration. Based on this what can you infer about the reactivity of helium and neon.
 Source: slideplayer.com
Source: slideplayer.com
The noble gases historically also the inert gases. Helium has 2 electrons filling its valence shell whereas neon. The nonreactive properties of helium arise from the fact that it is the lightest of the generally nonreactive noble gases a noble gas has a full electron shell meaning that it cannot easily give or receive electrons in a chemical reaction. Helium does not react with water. Helium was first observed by studying the sun and was named after the greek word for the sun helios.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
Based on this what can you infer about the reactivity of helium and neon. This difference in abundances can be observed in the earth s atmosphere where the ratio of 4 he atoms to 3 he atoms is approximately 1000000 1. Sometimes referred to as aerogens make up a class of chemical elements with similar properties. Atoms react and form chemical bonds with other atoms. It does not react with any other elements or ions so there are no helium bearing minerals in nature.
 Source: sciencewithme.com
Source: sciencewithme.com
I can infer that helium and neon are not reactive. Sun is a chemical element with the symbol he and atomic number 2. Based on this what can you infer about the reactivity of helium and neon. The noble gases historically also the inert gases. Reaction of helium with water.
 Source: slideplayer.com
Source: slideplayer.com
Helium has 2 electrons filling its valence shell whereas neon. Electron exchange or sharing is the basis for most chemical reactions so the noble gases tend to. Since it s completely full it s not happy with sharing or ceding electrons. Helium has 2 electrons filling its valence shell whereas neon. The abundance of helium 3 and helium 4 corresponds to 0 0002 and 99 9998 respectively.
 Source: quora.com
Source: quora.com
How does this row reflect the subshells of the second shell. Reaction of helium with water. Helium does not react with air even under extreme conditions. Helium has two known stable isotopes 3 he and 4 he. It does however dissolve slightly to the extent of about 8 61 cm 3 kg 1 at 20 c 293 k.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Since it s completely full it s not happy with sharing or ceding electrons. It does not react with any other elements or ions so there are no helium bearing minerals in nature. They re nobile gases meaning they have their outer valence electron shells filled and so practically no reactivity with other atoms. Electron exchange or sharing is the basis for most chemical reactions so the noble gases tend to. Helium was first observed by studying the sun and was named after the greek word for the sun helios.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title reactivity of helium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







