Soapy water surface tension
Soapy Water Surface Tension. The term surface tension is defined as the thin layer of force that holds water molecules together on the top. This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. In order for water to flow more easily into these small spaces you need to decrease its surface tension. Dip the dropper into the cup and suction in some water.
 Detergents Soaps And Surface Tension Experiment Rsc Education From edu.rsc.org
Detergents Soaps And Surface Tension Experiment Rsc Education From edu.rsc.org
Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. The effect of adding an unrelated chemical to a substance and thereby changing its surface tension is demonstrated by the example of putting soap a surfactant in water to reduce the surface. This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. This is one of the reasons why soap makes a good cleaner. Place drops of water from the dropper onto a penny counting how many it takes for the water to spill off the penny. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water.
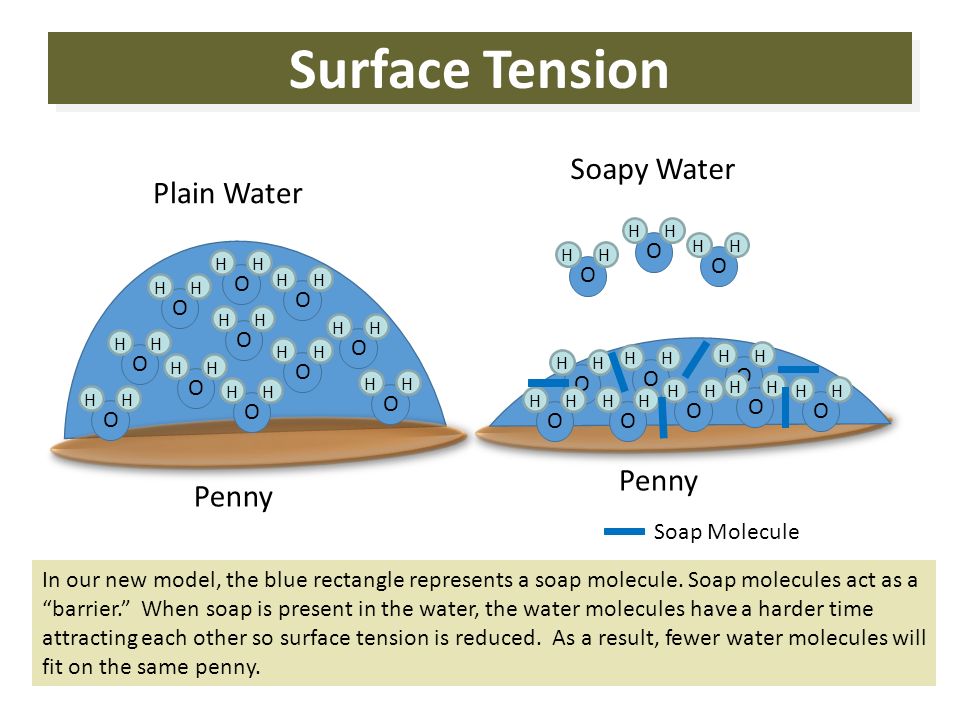
When soap is added the surface tension is reduced and the water wants to spread out flat water normally bulges up slightly like when you overfill a glass of water or if you have a single drop of water sitting proud on a table top.
This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. When soap is added the surface tension is reduced and the water wants to spread out flat water normally bulges up slightly like when you overfill a glass of water or if you have a single drop of water sitting proud on a table top. This is the number of drops it takes until the surface tension breaks. Students will employ the scientific method by developing a hypothesis testing the hypothesis by collecting and analyzing data and drawing a conclusion. Trial 2 trial 3 trial 4 trial 5 a average tap water soapy water 4. Analyze the data and draw conclusions.
 Source: arch.mcgill.ca
Source: arch.mcgill.ca
This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. In order for water to flow more easily into these small spaces you need to decrease its surface tension. Fill a plastic cup with water. Place drops of water from the dropper onto a penny counting how many it takes for the water to spill off the penny. Surface tension allows insects e g.
 Source: sciencebuddies.org
Source: sciencebuddies.org
You can do this by adding soap which is a surfactant a material that decreases the. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. Analyze the data and draw conclusions. Surface tension allows insects e g. At liquid air interfaces surface tension results from the greater attraction of liquid molecules to each other due to cohesion than to the molecules in the.
 Source: csun.edu
Source: csun.edu
This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. Support or reject your hypothesis. Dip the dropper into the cup and suction in some water. The term surface tension is defined as the thin layer of force that holds water molecules together on the top. Students will employ the scientific method by developing a hypothesis testing the hypothesis by collecting and analyzing data and drawing a conclusion.
 Source: labman.phys.utk.edu
Source: labman.phys.utk.edu
Suggest a reason for your observations why did it happen. Analyze the data and draw conclusions. This is one of the reasons why soap makes a good cleaner. This is the number of drops it takes until the surface tension breaks. The dependant variable in this experiment was the numbers of drops of water that varied vastly between the water and soapy water.
 Source: prezi.com
Source: prezi.com
Soap reduces surface tension. In order to answer this question fully you must first understand what the word really means. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. In order for water to flow more easily into these small spaces you need to decrease its surface tension. Write a paragraph below that explains how soap affects the surface tension of water using your data to help you answer the question.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Soap reduces surface tension. Soap reduces surface tension. The term surface tension is defined as the thin layer of force that holds water molecules together on the top. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. Analyze the data and draw conclusions.
 Source: researchgate.net
Source: researchgate.net
In order for water to flow more easily into these small spaces you need to decrease its surface tension. Write a paragraph below that explains how soap affects the surface tension of water using your data to help you answer the question. Support or reject your hypothesis. The term surface tension is defined as the thin layer of force that holds water molecules together on the top. At liquid air interfaces surface tension results from the greater attraction of liquid molecules to each other due to cohesion than to the molecules in the.
 Source: edu.rsc.org
Source: edu.rsc.org
As it spreads out it flattens on the dish and carries any pepper that s floating on the surface with it away from the source of the soap and to the edge of the water. The dependant variable in this experiment was the numbers of drops of water that varied vastly between the water and soapy water. Fill a plastic cup with water. This is the number of drops it takes until the surface tension breaks. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible.
 Source: edu.rsc.org
Source: edu.rsc.org
In order for water to flow more easily into these small spaces you need to decrease its surface tension. This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. Suggest a reason for your observations why did it happen. Fill a plastic cup with water. Analyze the data and draw conclusions.
 Source: comsol.com
Source: comsol.com
As it spreads out it flattens on the dish and carries any pepper that s floating on the surface with it away from the source of the soap and to the edge of the water. This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. In order for water to flow more easily into these small spaces you need to decrease its surface tension. This is one of the reasons why soap makes a good cleaner.
 Source: slideplayer.com
Source: slideplayer.com
Trial 2 trial 3 trial 4 trial 5 a average tap water soapy water 4. When soap is added the surface tension is reduced and the water wants to spread out flat water normally bulges up slightly like when you overfill a glass of water or if you have a single drop of water sitting proud on a table top. Suggest a reason for your observations why did it happen. Support or reject your hypothesis. The dependant variable in this experiment was the numbers of drops of water that varied vastly between the water and soapy water.
 Source: yellow-scope.com
Source: yellow-scope.com
At liquid air interfaces surface tension results from the greater attraction of liquid molecules to each other due to cohesion than to the molecules in the. If soap increases the surface tension more drops of soapy water than tap water will stick to the penny whereas if soap decreases the surface tension fewer drops of soapy water will stick. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water. This is one of the reasons why soap makes a good cleaner. Surface tension allows insects e g.
 Source: slideserve.com
Source: slideserve.com
The effect of adding an unrelated chemical to a substance and thereby changing its surface tension is demonstrated by the example of putting soap a surfactant in water to reduce the surface. Water striders to float and slide on a water surface without becoming even partly submerged. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. Dip the dropper into the cup and suction in some water. The dependant variable in this experiment was the numbers of drops of water that varied vastly between the water and soapy water.
 Source: prezi.com
Source: prezi.com
This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. This makes soap an effective cleaner which can go through the minute pores of the clothes with ease. Dip the dropper into the cup and suction in some water. In order to answer this question fully you must first understand what the word really means. This is the independent variable because the soap was added to the water changing the composition and surface tension of the water.
 Source: sites.udel.edu
Source: sites.udel.edu
As it spreads out it flattens on the dish and carries any pepper that s floating on the surface with it away from the source of the soap and to the edge of the water. Students will employ the scientific method by developing a hypothesis testing the hypothesis by collecting and analyzing data and drawing a conclusion. This is the number of drops it takes until the surface tension breaks. As it spreads out it flattens on the dish and carries any pepper that s floating on the surface with it away from the source of the soap and to the edge of the water. This is one of the reasons why soap makes a good cleaner.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title soapy water surface tension by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







