Tin on periodic table
Tin On Periodic Table. Its chief ore is cassiterite an oxide. Tin has two allotropes forms. Soft malleable and resistant to corrosion tin is used as a protective coating for iron steel copper and other metals and in such alloys as solder pewter bronze and type metal. It has 50 protons and 50 electrons in the atomic structure.
 Sn Element Tin Latin Name Atomic Data Uses Health Hazards With Videos From byjus.com
Sn Element Tin Latin Name Atomic Data Uses Health Hazards With Videos From byjus.com
Chemical element in the periodic table of elements. The chemical symbol for tin is sn. Tin periodic table. Element tin sn group 14 atomic number 50 p block mass 118 710. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn. The most abundant is 118 sn at 24.
Of these ten are stable.
Soft malleable and resistant to corrosion tin is used as a protective coating for iron steel copper and other metals and in such alloys as solder pewter bronze and type metal. The most abundant is 118 sn at 24. Tin has atomic number 50 and its atomic weight is 118 69. Element tin sn group 14 atomic number 50 p block mass 118 710. It is obtained chiefly from the mineral cassiterite which contains stannic oxide sno 2. 112 sn 114 sn 115 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn.
![]() Source: vectorstock.com
Source: vectorstock.com
Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. Sources facts uses scarcity sri podcasts alchemical symbols videos and images. Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. Tin is a 50. It is obtained chiefly from the mineral cassiterite which contains stannic oxide sno 2.
 Source: shop.spreadshirt.net
Source: shop.spreadshirt.net
Tin has two allotropes forms. Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. The chemical symbol for tin is sn. Chemical element in the periodic table of elements. Tin is used as oxidation resistant coating material due to its low melting point.
 Source: diarystore.com
Source: diarystore.com
Tin has atomic number 50 and its atomic weight is 118 69. Soft malleable and resistant to corrosion tin is used as a protective coating for iron steel copper and other metals and in such alloys as solder pewter bronze and type metal. Tin has two allotropes forms. Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. Of these ten are stable.
![]() Source: vectorstock.com
Source: vectorstock.com
Of these ten are stable. The most abundant is 118 sn at 24. Tin has atomic number 50 and its atomic weight is 118 69. It has 50 protons and 50 electrons in the atomic structure. Its chief ore is cassiterite an oxide.
 Source: chemistrylearner.com
Source: chemistrylearner.com
β tin is silvery white soft metal and at low temperature it transforms into less dense α tin metal which is gray in color and has diamond cubic structure 2. β tin is silvery white soft metal and at low temperature it transforms into less dense α tin metal which is gray in color and has diamond cubic structure 2. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn. Tin shows a chemical similarity to both of its neighbors in group 14 germanium and lead and has two main oxidation states 2 and the slightly more stable 4. It is obtained chiefly from the mineral cassiterite which contains stannic oxide sno 2.
 Source: byjus.com
Source: byjus.com
35 whose half lives are known mass numbers 100 to 134. Element tin sn group 14 atomic number 50 p block mass 118 710. Tin periodic table. Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. 35 whose half lives are known mass numbers 100 to 134.
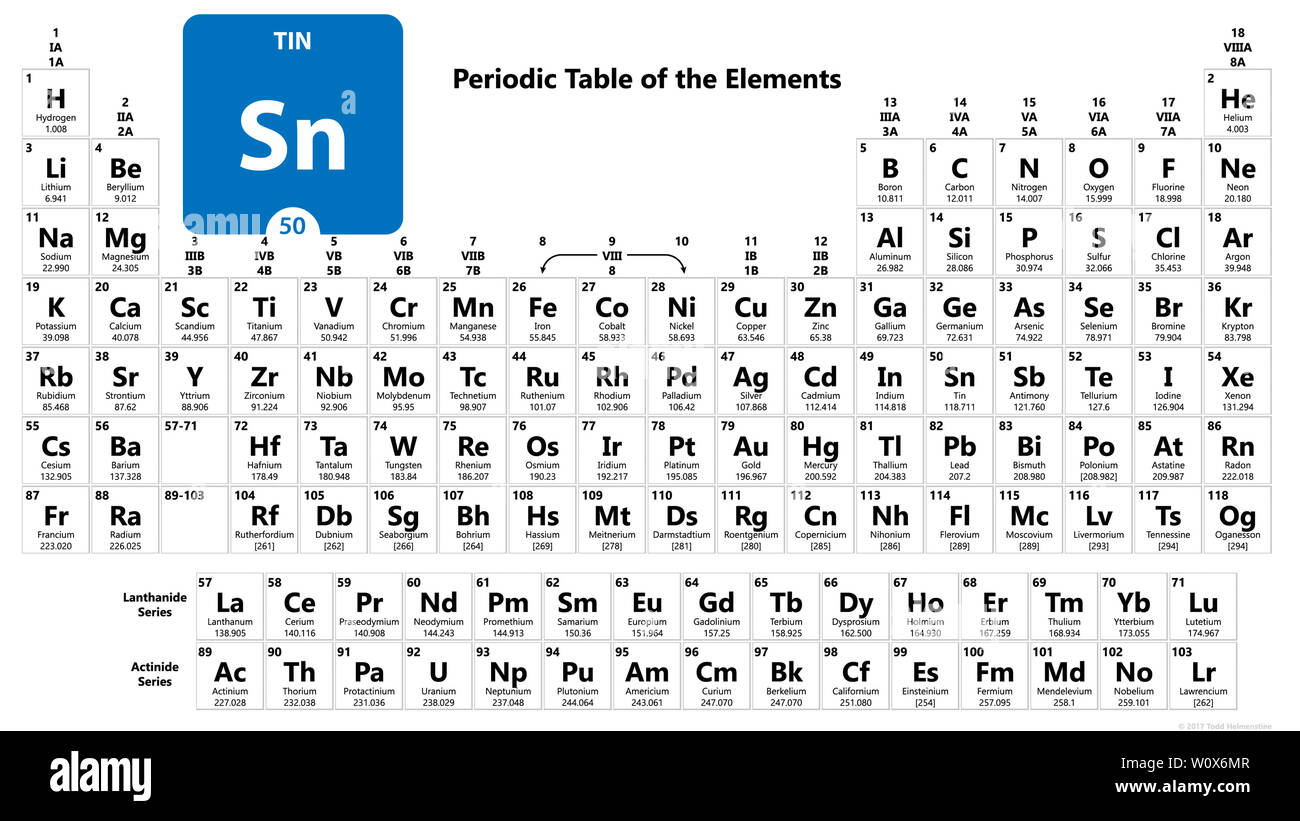 Source: alamy.com
Source: alamy.com
Tin is a 50. The most abundant is 118 sn at 24. Tin has two allotropes forms. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn. Chemical element in the periodic table of elements.
 Source: knowledgedoor.com
Source: knowledgedoor.com
Of these ten are stable. Of these ten are stable. β tin is silvery white soft metal and at low temperature it transforms into less dense α tin metal which is gray in color and has diamond cubic structure 2. Its chief ore is cassiterite an oxide. It has 50 protons and 50 electrons in the atomic structure.
 Source: canstockphoto.com
Source: canstockphoto.com
Tin periodic table. Soft malleable and resistant to corrosion tin is used as a protective coating for iron steel copper and other metals and in such alloys as solder pewter bronze and type metal. The chemical symbol for tin is sn. Of these ten are stable. Tin is a 50.
 Source: alamy.com
Source: alamy.com
Tin is a 50. Tin is a 50. Tin shows a chemical similarity to both of its neighbors in group 14 germanium and lead and has two main oxidation states 2 and the slightly more stable 4. β tin is silvery white soft metal and at low temperature it transforms into less dense α tin metal which is gray in color and has diamond cubic structure 2. 112 sn 114 sn 115 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn.
 Source: cafepress.com
Source: cafepress.com
Its chief ore is cassiterite an oxide. Tin is used as oxidation resistant coating material due to its low melting point. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn. Tin periodic table. Of these ten are stable.
 Source: britannica.com
Source: britannica.com
It has 50 protons and 50 electrons in the atomic structure. It has 50 protons and 50 electrons in the atomic structure. Tin shows a chemical similarity to both of its neighbors in group 14 germanium and lead and has two main oxidation states 2 and the slightly more stable 4. 35 whose half lives are known mass numbers 100 to 134. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn.
 Source: knowledgedoor.com
Source: knowledgedoor.com
The most abundant is 118 sn at 24. Tin is a 50. Tin is used as oxidation resistant coating material due to its low melting point. Chemical element in the periodic table of elements. Its chief ore is cassiterite an oxide.
 Source: bbc.co.uk
Source: bbc.co.uk
It is obtained chiefly from the mineral cassiterite which contains stannic oxide sno 2. Tin symbol sn metalloid element of group iv of the periodic table known from ancient times. Tin has two allotropes forms. Tin has atomic number 50 and its atomic weight is 118 69. It has 50 protons and 50 electrons in the atomic structure.
 Source: 123rf.com
Source: 123rf.com
It is obtained chiefly from the mineral cassiterite which contains stannic oxide sno 2. Tin facts isotopes there are ten naturally occurring isotopes of tin exist 112 sn 113 sn 114 sn 116 sn 117 sn 118 sn 119 sn 120 sn 122 sn and 124 sn. Its chief ore is cassiterite an oxide. Tin is a 50. The chemical symbol for tin is sn.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin on periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.