Vacuum distillation setup
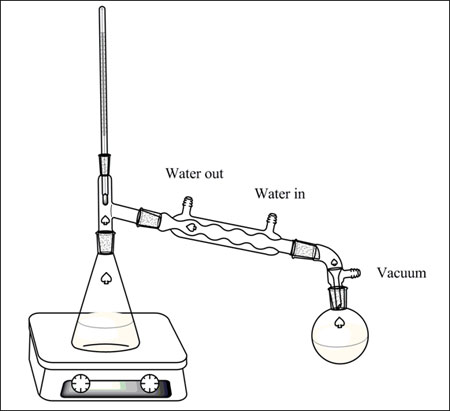
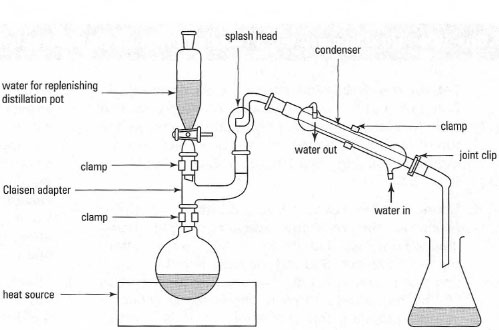
Vacuum Distillation Setup. Vacuum distillation is distillation performed under reduced pressure which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. A fraction distillation can also be used. Vacuum distillation is sometimes used in fire refining. Here is all the glassware properly assembled.
 The Scheme For The Vacuum Distillation Apparatus Of Aptes The Download Scientific Diagram From researchgate.net
The Scheme For The Vacuum Distillation Apparatus Of Aptes The Download Scientific Diagram From researchgate.net
In this process molten tin is heated in a dense graphite vessel at high temperatures 1 100 to 1 300 c or 2 000 to 2 375 f. The next items to be added are the thermometer adaptor and thermometer. A vacuum is applied and impurities are removed by selective distillation at their. It is assumed that readers have previously performed a simple distillation under atmospheric pressure so in this section are described differences between atmospheric and reduced pressure distillations. I already have standard lab glassware with 24 40 joints 10mm barbed inlets. Vacuum distillation is distillation performed under reduced pressure which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy.
This will ensure that there is no movement in the joints and no vapour can escape.
This technique separates compounds based on differences in boiling points. It is assumed that readers have previously performed a simple distillation under atmospheric pressure so in this section are described differences between atmospheric and reduced pressure distillations. Pig type receiving adapter condenser elastic band ensuring a tight seal can be helped by the use of using elastic bands as. In vacuum membrane distillation vacuum is applied in the permeate side and the condensation of molecules takes place outside the module figure 13 among the four md configurations described vmd exhibits the highest driving force at constant feed temperature and therefore the highest transmembrane flux. This will ensure that there is no movement in the joints and no vapour can escape. Arrows show direction of suction.
 Source: researchgate.net
Source: researchgate.net
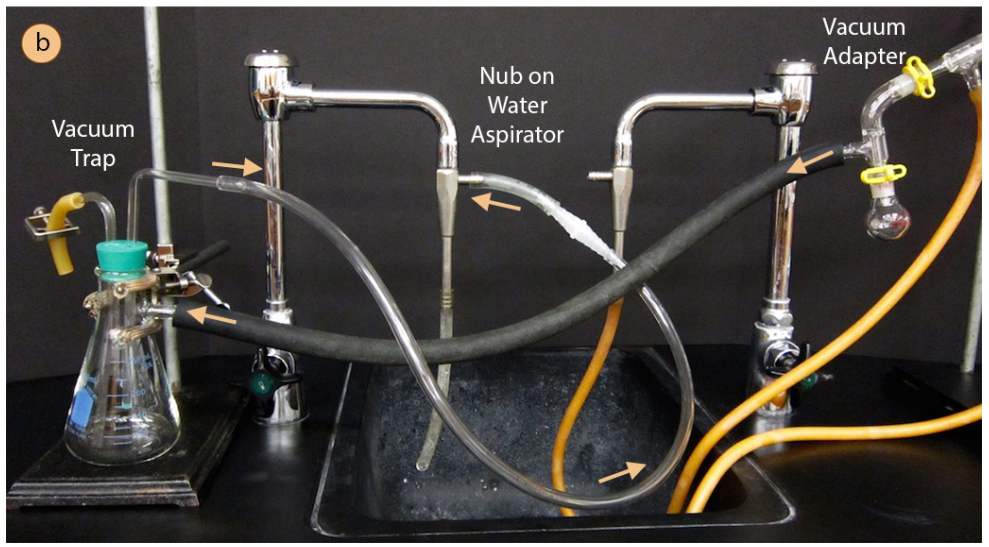
When setting up a vacuum distillation it is important to ensure that all of the ground glass joints are clipped and clamped firmly. A vacuum distillation setup b apparatus connected to a vacuum trap and water aspirator. Vacuum distillation is distillation performed under reduced pressure which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. The thermometer is always added last because it is large and susceptible to breakeage. Pig type receiving adapter condenser elastic band ensuring a tight seal can be helped by the use of using elastic bands as.
 Source: youtube.com
Source: youtube.com
The next items to be added are the thermometer adaptor and thermometer. A vacuum distillation apparatus is shown in figure 5 50 using a simple distillation setup. A vacuum distillation setup b apparatus connected to a vacuum trap and water aspirator. A vacuum is applied and impurities are removed by selective distillation at their. I already have standard lab glassware with 24 40 joints 10mm barbed inlets.
 Source: pinterest.com
Source: pinterest.com
I already have standard lab glassware with 24 40 joints 10mm barbed inlets. The reduction in boiling point can be c. Vacuum distillation is sometimes used in fire refining. In vacuum membrane distillation vacuum is applied in the permeate side and the condensation of molecules takes place outside the module figure 13 among the four md configurations described vmd exhibits the highest driving force at constant feed temperature and therefore the highest transmembrane flux. Arrows show direction of suction.
 Source: biocyclopedia.com
Source: biocyclopedia.com
In vacuum membrane distillation vacuum is applied in the permeate side and the condensation of molecules takes place outside the module figure 13 among the four md configurations described vmd exhibits the highest driving force at constant feed temperature and therefore the highest transmembrane flux. In vacuum membrane distillation vacuum is applied in the permeate side and the condensation of molecules takes place outside the module figure 13 among the four md configurations described vmd exhibits the highest driving force at constant feed temperature and therefore the highest transmembrane flux. In a vacuum distillation a round bottom flask is used as the receiving flask and it is securely attached with either a clamp or a yellow clip. In this process molten tin is heated in a dense graphite vessel at high temperatures 1 100 to 1 300 c or 2 000 to 2 375 f. Arrows show direction of suction.
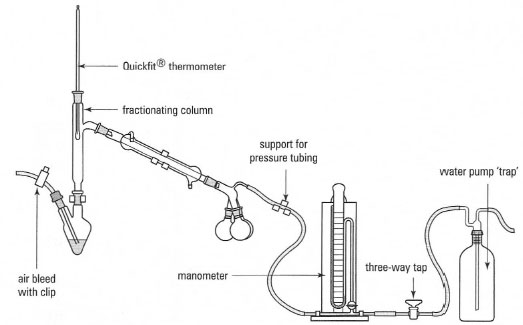
 Source: designer-drug.com
Source: designer-drug.com
A reduced pressure decreases the boiling point of compounds. Vacuum distillation is sometimes used in fire refining. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. This technique separates compounds based on differences in boiling points. Pig type receiving adapter condenser elastic band ensuring a tight seal can be helped by the use of using elastic bands as.
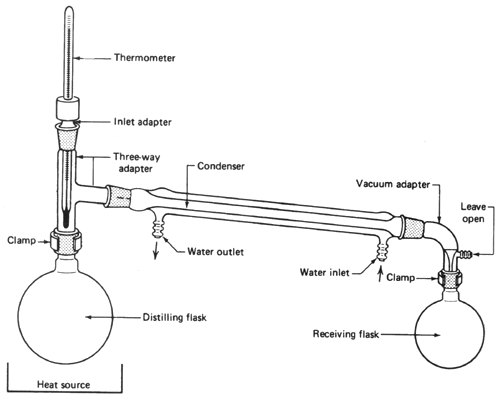
 Source: cbn.cambridgesoft.com
Source: cbn.cambridgesoft.com
The thermometer is always added last because it is large and susceptible to breakeage. A vacuum distillation setup b apparatus connected to a vacuum trap and water aspirator. A fraction distillation can also be used. Here is all the glassware properly assembled. In this process molten tin is heated in a dense graphite vessel at high temperatures 1 100 to 1 300 c or 2 000 to 2 375 f.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Here is all the glassware properly assembled. I already have standard lab glassware with 24 40 joints 10mm barbed inlets. A vacuum is applied and impurities are removed by selective distillation at their. It is assumed that readers have previously performed a simple distillation under atmospheric pressure so in this section are described differences between atmospheric and reduced pressure distillations. Arrows show direction of suction.
 Source: youtube.com
Source: youtube.com
A vacuum distillation apparatus is shown in figure 5 50 using a simple distillation setup. I want to assemble a hobbyist level vacuum distillation apparatus for extracting small volumes of typical solvents e g water alcohols ketones diols. Here is all the glassware properly assembled. A vacuum distillation apparatus is shown in figure 5 50 using a simple distillation setup. A fraction distillation can also be used.
 Source: umsl.edu
Source: umsl.edu
A fraction distillation can also be used. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. I want to assemble a hobbyist level vacuum distillation apparatus for extracting small volumes of typical solvents e g water alcohols ketones diols. It is assumed that readers have previously performed a simple distillation under atmospheric pressure so in this section are described differences between atmospheric and reduced pressure distillations. A fraction distillation can also be used.
 Source: sciencedirect.com
Source: sciencedirect.com
A vacuum is applied and impurities are removed by selective distillation at their. A fraction distillation can also be used. A vacuum is applied and impurities are removed by selective distillation at their. In vacuum membrane distillation vacuum is applied in the permeate side and the condensation of molecules takes place outside the module figure 13 among the four md configurations described vmd exhibits the highest driving force at constant feed temperature and therefore the highest transmembrane flux. A vacuum distillation setup b apparatus connected to a vacuum trap and water aspirator.
 Source: biocyclopedia.com
Source: biocyclopedia.com
Arrows show direction of suction. Vacuum distillation is distillation performed under reduced pressure which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. The thermometer is always added last because it is large and susceptible to breakeage. A vacuum is applied and impurities are removed by selective distillation at their. This will ensure that there is no movement in the joints and no vapour can escape.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The next items to be added are the thermometer adaptor and thermometer. But since i have no idea how to set up a vacuum distillation i will invite any learned man to come and share some do s and dont s on how to get that sweet low temp boil without wrecking 1500 worth of glassware reply with quote. Here is all the glassware properly assembled. I already have standard lab glassware with 24 40 joints 10mm barbed inlets. Pig type receiving adapter condenser elastic band ensuring a tight seal can be helped by the use of using elastic bands as.
 Source: schoolbag.info
Source: schoolbag.info
A vacuum is applied and impurities are removed by selective distillation at their. But since i have no idea how to set up a vacuum distillation i will invite any learned man to come and share some do s and dont s on how to get that sweet low temp boil without wrecking 1500 worth of glassware reply with quote. In a vacuum distillation a round bottom flask is used as the receiving flask and it is securely attached with either a clamp or a yellow clip. The next items to be added are the thermometer adaptor and thermometer. A fraction distillation can also be used.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A vacuum distillation apparatus is shown in figure 5 50 using a simple distillation setup. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. This will ensure that there is no movement in the joints and no vapour can escape. It is assumed that readers have previously performed a simple distillation under atmospheric pressure so in this section are described differences between atmospheric and reduced pressure distillations. A fraction distillation can also be used.
 Source: chem.libretexts.org
Source: chem.libretexts.org
I want to assemble a hobbyist level vacuum distillation apparatus for extracting small volumes of typical solvents e g water alcohols ketones diols. The reduction in boiling point can be c. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. A reduced pressure decreases the boiling point of compounds. I want to assemble a hobbyist level vacuum distillation apparatus for extracting small volumes of typical solvents e g water alcohols ketones diols.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vacuum distillation setup by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.