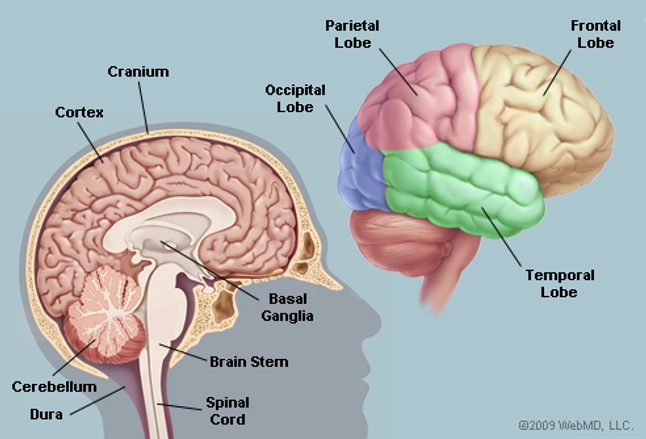
Very reactive metals
Very Reactive Metals. The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. The most reactive metal on the periodic table is francium. It ignites when heated and burns with a brilliant white flame other metals may be more reactive than magnesium or in between magnesium and platinum. Lilac flame and white solid.
 What Are Reactivity Series Definition From Seneca Learning From senecalearning.com
What Are Reactivity Series Definition From Seneca Learning From senecalearning.com
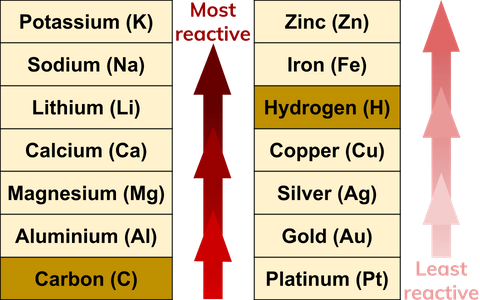
The most reactive metal is francium the last alkali metal and most expensive element. Lilac flame and white solid. The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. All oxides produced under the first two metal groups can resist hydrogen ion reduction. Potassium k reacts vigorous when heated. In the chart the first five elements are considered highly reactive.
The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides.
Potassium k reacts vigorous when heated. The halogens alkali metals and alkaline earth metals are highly reactive. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. All oxides produced under the first two metal groups can resist hydrogen ion reduction. It ignites when heated and burns with a brilliant white flame other metals may be more reactive than magnesium or in between magnesium and platinum. Lilac flame and white solid.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. In the chart the first five elements are considered highly reactive. The most reactive metal is francium the last alkali metal and most expensive element. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. Cesium reacts explosively with water though it is predicted francium would react even more vigorously.
 Source: modelscience.com
Source: modelscience.com
The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. All oxides produced under the first two metal groups can resist hydrogen ion reduction. The most reactive metal on the periodic table is francium. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. The most reactive element is fluorine the first element in the halogen group.
 Source: compoundchem.com
Source: compoundchem.com
Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. In the chart the first five elements are considered highly reactive. The most reactive metal on the periodic table is francium. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Magnesium is very reactive.
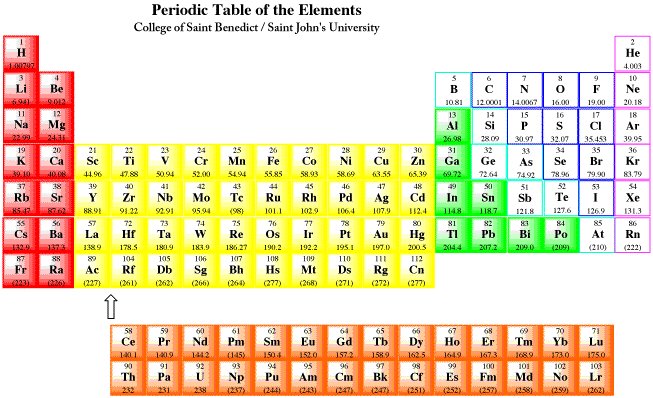 Source: employees.csbsju.edu
Source: employees.csbsju.edu
The most reactive metal on the periodic table is francium. Magnesium is very reactive. Lilac flame and white solid. The most reactive element is fluorine the first element in the halogen group. The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The most reactive element is fluorine the first element in the halogen group. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. The most reactive metal on the periodic table is francium. Potassium k reacts vigorous when heated. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium.
Source: quora.com
The most reactive metal is francium the last alkali metal and most expensive element. All oxides produced under the first two metal groups can resist hydrogen ion reduction. Potassium k reacts vigorous when heated. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. The halogens alkali metals and alkaline earth metals are highly reactive.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
All oxides produced under the first two metal groups can resist hydrogen ion reduction. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. All oxides produced under the first two metal groups can resist hydrogen ion reduction. The most reactive metal is francium the last alkali metal and most expensive element. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas.
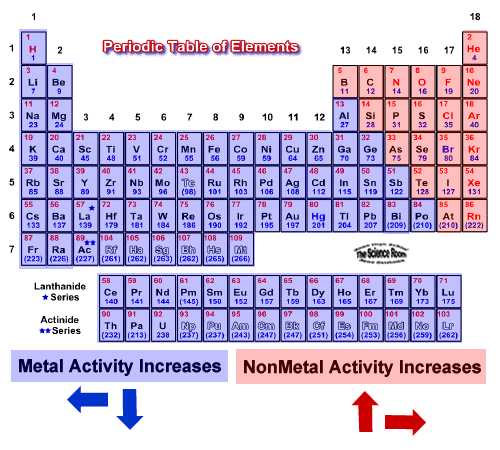 Source: socratic.org
Source: socratic.org
The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. All oxides produced under the first two metal groups can resist hydrogen ion reduction. Potassium k reacts vigorous when heated. Reaction with oxygen when heated and at room temperature reaction with water. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas.
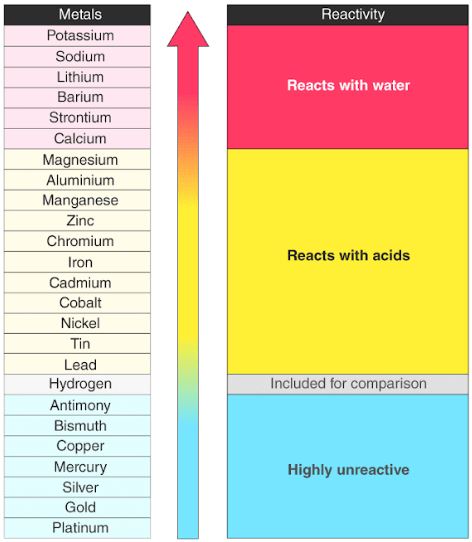 Source: byjus.com
Source: byjus.com
Magnesium is very reactive. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. Lilac flame and white solid. All oxides produced under the first two metal groups can resist hydrogen ion reduction. Reaction with oxygen when heated and at room temperature reaction with water.

Cesium reacts explosively with water though it is predicted francium would react even more vigorously. The most reactive element is fluorine the first element in the halogen group. In the chart the first five elements are considered highly reactive. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Reaction with oxygen when heated and at room temperature reaction with water.
 Source: jagranjosh.com
Source: jagranjosh.com
Magnesium is very reactive. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. Lilac flame and white solid. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. The most reactive element is fluorine the first element in the halogen group.
 Source: britannica.com
Source: britannica.com
Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Magnesium is very reactive. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. The most reactive metal on the periodic table is francium. The most reactive metal is francium the last alkali metal and most expensive element.
 Source: toppr.com
Source: toppr.com
It ignites when heated and burns with a brilliant white flame other metals may be more reactive than magnesium or in between magnesium and platinum. The most reactive metal is francium the last alkali metal and most expensive element. The most reactive element is fluorine the first element in the halogen group. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. Lilac flame and white solid.
 Source: pinterest.co.uk
Source: pinterest.co.uk
In the chart the first five elements are considered highly reactive. The most reactive metal is francium the last alkali metal and most expensive element. These can react with hot water or cold water and form a steam with hydroxides and hydrogen gas. Reaction with oxygen when heated and at room temperature reaction with water. Potassium k reacts vigorous when heated.
 Source: senecalearning.com
Source: senecalearning.com
The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. In the chart the first five elements are considered highly reactive. The next four metals following the list from magnesium to chromium are regarded as active metals that that can form a reaction with hot water or steam in order to produce hydrogen gas and oxides. Magnesium is very reactive. The most reactive element is fluorine the first element in the halogen group.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title very reactive metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.