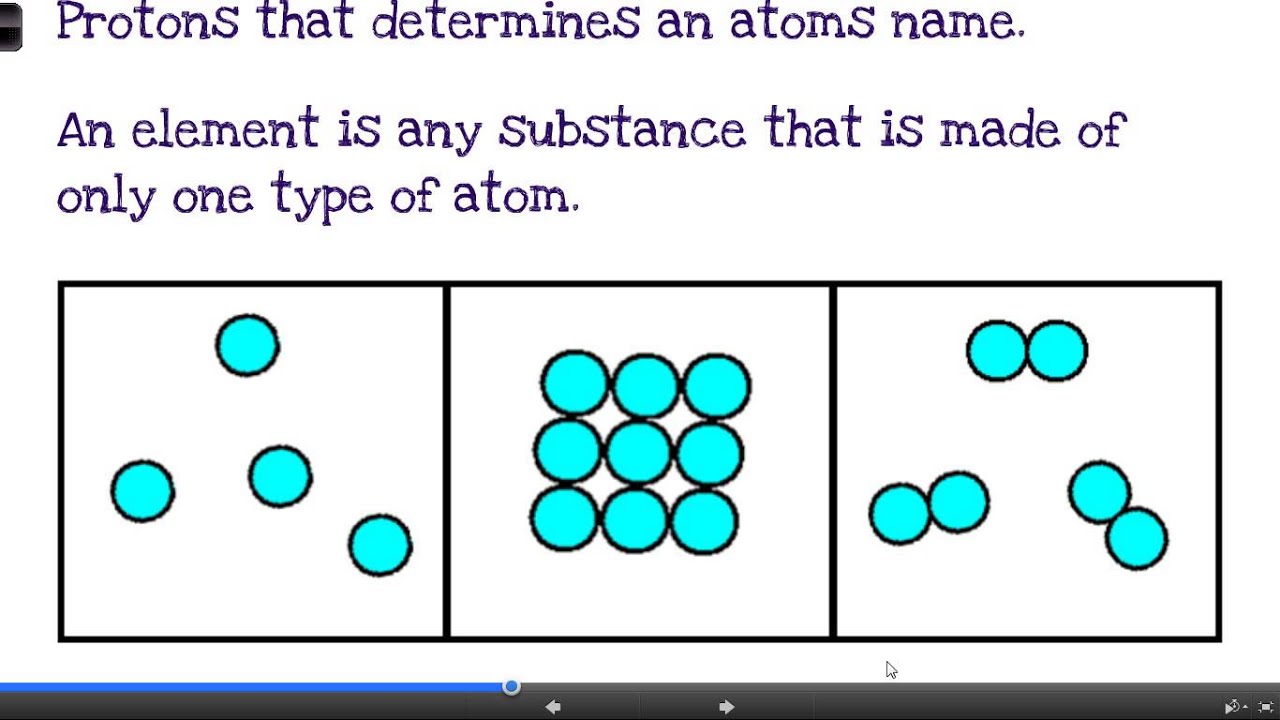
What are elements compounds and mixtures
What Are Elements Compounds And Mixtures. Carbon dioxide is up of carbon and oxygen. This topic is school chemistry pre gcse. For example water can be vapor solid or the usual liquid. Copper cu oxygen o hydrogen h helium he lithium li b compound.
 Element Compound Mixture Worksheet Awesome Elements Pounds Mixtures Handout By Cs Compounds And Mixtures Elements Compounds And Mixtures Chemistry Worksheets From pinterest.com
Element Compound Mixture Worksheet Awesome Elements Pounds Mixtures Handout By Cs Compounds And Mixtures Elements Compounds And Mixtures Chemistry Worksheets From pinterest.com
Elements compounds and mixtures 1. All compounds are substances but not all substances are compounds. This topic is school chemistry pre gcse. A compound is a substance which contains two or more elements chemically combined together. Therefore it has a definite chemical composition. Elements are represented by symbols.
Matter can be placed into two classes.
Mixtures are considered impure substances because no new substance is formed therefore they do not have any fixed properties. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. Mixtures can be separated more easily than compounds. Using composition to describe matter is better than using its state because the internal makeup makes matter unique and not its phase or state. Water is made up of hydrogen and oxygen atoms.
 Source: storyboardthat.com
Source: storyboardthat.com
Compounds can be classified as pure substances because each element that formed it is in fixed proportions. Compounds can be classified as pure substances because each element that formed it is in fixed proportions. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Elements are represented by symbols. Mixtures can be separated more easily than compounds.
 Source: youtube.com
Source: youtube.com
This topic is school chemistry pre gcse. There are many different methods of separating mixtures depending on the properties of the substances in the mixture and whether it is a. Matter can be placed into two classes. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Pure substances and mixtures.
 Source: youtube.com
Source: youtube.com
Microscopic view of a gaseous mixture containing two elements argon and nitrogen and a compound water. Matter can be placed into two classes. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Note that a mixture. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged.
 Source: pinterest.com
Source: pinterest.com
A compound is a substance which contains two or more elements chemically combined together. Pure substances and mixtures. This classification is based on the internal composition of that matter. Two or more elements combined into one substance through a chemical reaction such as water form a chemical compound. This topic is school chemistry pre gcse.
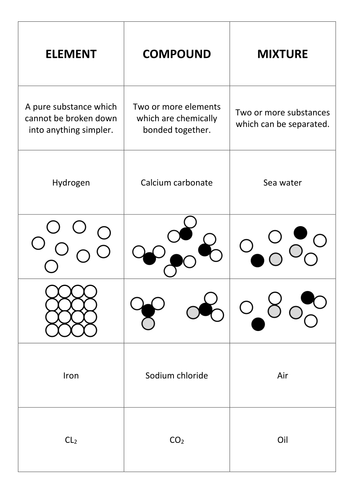
Elements mixtures and compounds are the names of types of chemicals. Carbon dioxide is up of carbon and oxygen. Matter can be placed into two classes. Pure substances and mixtures. There are many different methods of separating mixtures depending on the properties of the substances in the mixture and whether it is a.
 Source: slideplayer.com
Source: slideplayer.com
Compounds can be classified as pure substances because each element that formed it is in fixed proportions. This classification is based on the internal composition of that matter. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Note that a mixture. A pure substance is a substance in which there is only one type of particle.
 Source: diffen.com
Source: diffen.com
Compounds can be classified as pure substances because each element that formed it is in fixed proportions. This topic is school chemistry pre gcse. Using composition to describe matter is better than using its state because the internal makeup makes matter unique and not its phase or state. Water is made up of hydrogen and oxygen atoms. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded.
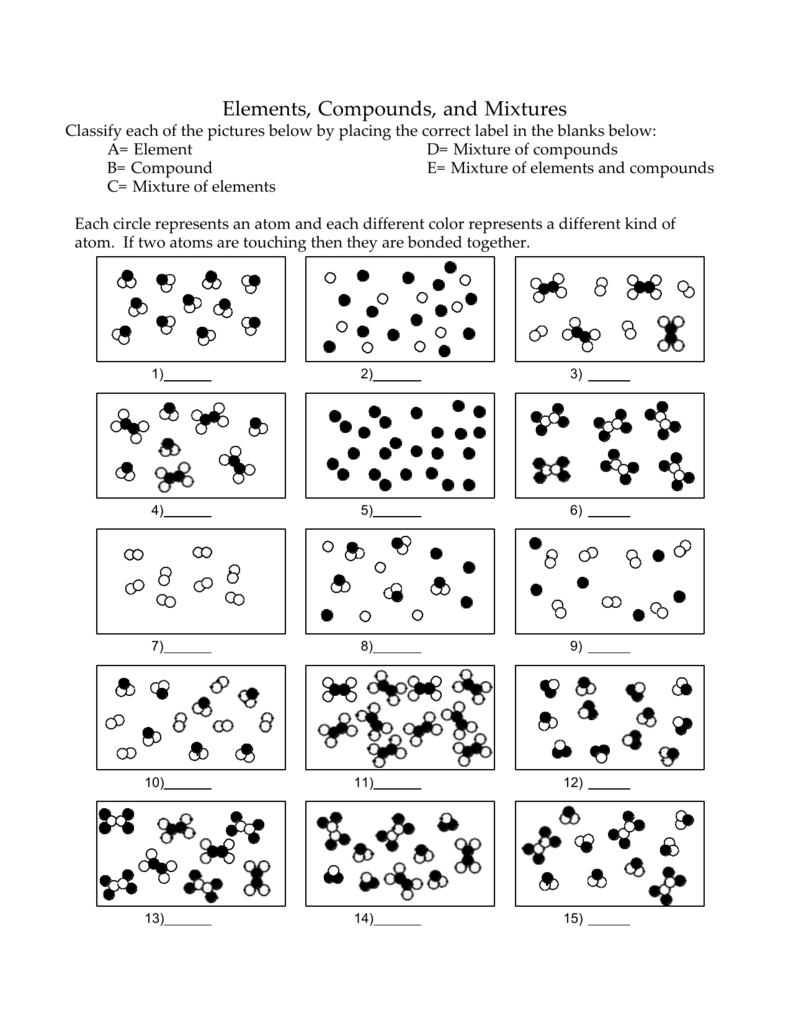 Source: studylib.net
Source: studylib.net
Copper cu oxygen o hydrogen h helium he lithium li b compound. Two or more elements combined into one substance through a chemical reaction such as water form a chemical compound. Mixtures can be separated more easily than compounds. A compound is a substance which contains two or more elements chemically combined together. Consists of two or more different elements and or compounds physically intermingled can be separated into its components by physical means and.

Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. Elements are pure substances so each element only contains one type of particle. Carbon dioxide is up of carbon and oxygen. Pure substances and mixtures. All compounds are substances but not all substances are compounds.
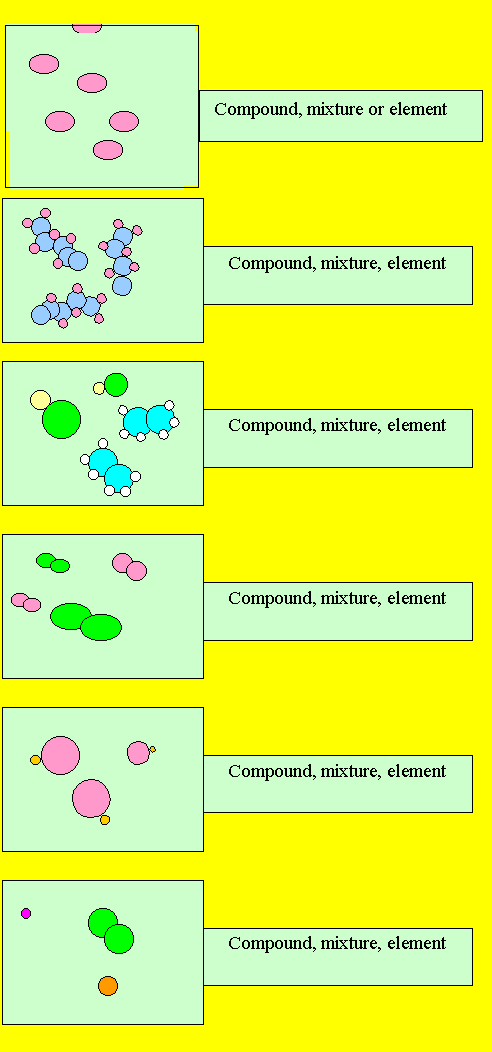 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Elements mixtures and compounds are the names of types of chemicals. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Water is made up of hydrogen and oxygen atoms. This topic is school chemistry pre gcse. Examples of mixtures are sea water air powdered iron powdered sulfur and most rocks.
 Source: open.edu
Source: open.edu
For example water can be vapor solid or the usual liquid. This classification is based on the internal composition of that matter. Using composition to describe matter is better than using its state because the internal makeup makes matter unique and not its phase or state. Mixtures can be any combination of elements and or compounds. Matter can be placed into two classes.
 Source: pinterest.com
Source: pinterest.com
Carbon dioxide is up of carbon and oxygen. Pure substances and mixtures. All compounds are substances but not all substances are compounds. For example water can be vapor solid or the usual liquid. There are many different methods of separating mixtures depending on the properties of the substances in the mixture and whether it is a.
 Source: proprofs.com
Source: proprofs.com
Mixtures can be any combination of elements and or compounds. Water is made up of hydrogen and oxygen atoms. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Carbon dioxide is up of carbon and oxygen. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3.
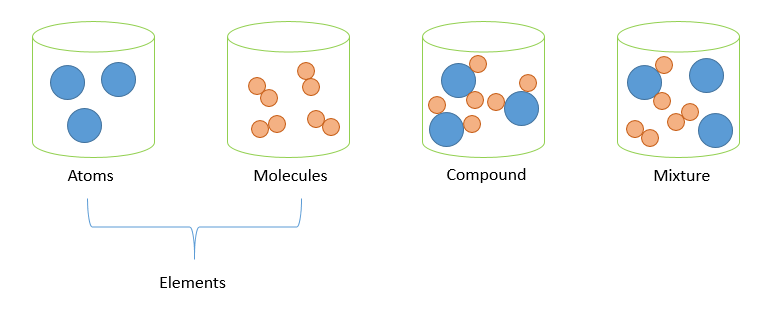 Source: minichemistry.com
Source: minichemistry.com
A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Examples of mixtures are sea water air powdered iron powdered sulfur and most rocks. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. Note that a mixture. A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded.
 Source: quizlet.com
Source: quizlet.com
A pure substance is a substance in which there is only one type of particle. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. All compounds are substances but not all substances are compounds. Mixtures are considered impure substances because no new substance is formed therefore they do not have any fixed properties. Mixtures can be any combination of elements and or compounds.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what are elements compounds and mixtures by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.