What are the least reactive metals found in pure form
What Are The Least Reactive Metals Found In Pure Form. Is the least reactive metal and they are often called the noble metals they can be found in pure form in nature some are used by ancient people to make jewellery and coins. Noble metals are found as pure metals because they are nonreactive and don t combine with other elements to form compounds. The extraction of the least reactive metals. Most native specimens contain traces of iron and it is likely to be found associated with other elements such as gold copper and nickel in alloyed form or laced with other rare metals.
 Metals On The Periodic Table Definition Reactivity Video Lesson Transcript Study Com From study.com
Metals On The Periodic Table Definition Reactivity Video Lesson Transcript Study Com From study.com
This makes them ideal for jewelry and coins. Wrought iron is iron that is pure or almost pure in particular containing very little carbon. Because they are so nonreactive they don t corrode easily. The more stable the compound the more difficult it is to extract the metals for it. The extraction of the least reactive metals. Is the least reactive metal and they are often called the noble metals they can be found in pure form in nature some are used by ancient people to make jewellery and coins.
The more stable the compound the more difficult it is to extract the metals for it.
Because they are so nonreactive they don t corrode easily. Wrought iron is iron that is pure or almost pure in particular containing very little carbon. Because they are so nonreactive they don t corrode easily. Least reactive metals in pure form. Noble metals are found as pure metals because they are nonreactive and don t combine with other elements to form compounds. The more stable the compound the more difficult it is to extract the metals for it.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The very least reactive metals such as gold occur as pure elements but may be contaminated with other elements. It is extremely rare to find platinum in pure form. What is the stability of the more reactive metals. Because they are so nonreactive they don t corrode easily. Gold silver titanium palladium platinum.
Source: quora.com
It is extremely rare to find platinum in pure form. The more reactive the more stable it is as a compound. The extraction of the least reactive metals. They are cleaned through various chemical reactions. It was used to make decorative items such as gates.
 Source: quora.com
Source: quora.com
Gold platinum iridium and rhodium. Five groups of metals. Least reactive metals usually occur in form of free metal. The more stable the compound the more difficult it is to extract the metals for it. Wrought iron is iron that is pure or almost pure in particular containing very little carbon.
 Source: quora.com
Source: quora.com
Five groups of metals. The very least reactive metals such as gold occur as pure elements but may be contaminated with other elements. Metals from middle order of reactivity series are found in form of oxides sulphides and carbonates. They tend to be found in their pure form so it is easy to extract mainly through displacement reactions. This makes them ideal for jewelry and coins.
 Source: study.com
Source: study.com
Platinum is the least reactive metal and exhibits remarkable resistance to corrosion even at high temperature making it extremely valuable for industrial use. The extraction of the least reactive metals. The very least reactive metals such as gold occur as pure elements but may be contaminated with other elements. Noble metals include copper palladium silver platinum and gold. Five groups of metals.
 Source: thechemistryguru.com
Source: thechemistryguru.com
Examples are gold silver and copper. Least reactive metals in pure form. It was used to make decorative items such as gates. What is the stability of the more reactive metals. They are cleaned through various chemical reactions.
 Source: ck12.org
Source: ck12.org
Most native specimens contain traces of iron and it is likely to be found associated with other elements such as gold copper and nickel in alloyed form or laced with other rare metals. The extraction of the least reactive metals. Metals from middle order of reactivity series are found in form of oxides sulphides and carbonates. It is no longer made on a large scale and the. Platinum is the least reactive metal and exhibits remarkable resistance to corrosion even at high temperature making it extremely valuable for industrial use.
Source: quora.com
Least reactive metals in pure form. Most native specimens contain traces of iron and it is likely to be found associated with other elements such as gold copper and nickel in alloyed form or laced with other rare metals. It is no longer made on a large scale and the. The extraction of the least reactive metals. Gold platinum iridium and rhodium.
 Source: ck12.org
Source: ck12.org
The more stable the compound the more difficult it is to extract the metals for it. Five groups of metals. They tend to be found in their pure form so it is easy to extract mainly through displacement reactions. Is the least reactive metal and they are often called the noble metals they can be found in pure form in nature some are used by ancient people to make jewellery and coins. The very least reactive metals such as gold occur as pure elements but may be contaminated with other elements.
 Source: toppr.com
Source: toppr.com
Because they are so nonreactive they don t corrode easily. Least reactive metals in pure form. Wrought iron is iron that is pure or almost pure in particular containing very little carbon. The more reactive the more stable it is as a compound. It is extremely rare to find platinum in pure form.
 Source: ck12.org
Source: ck12.org
It was used to make decorative items such as gates. Platinum is the least reactive metal and exhibits remarkable resistance to corrosion even at high temperature making it extremely valuable for industrial use. It was used to make decorative items such as gates. Gold silver titanium palladium platinum. Is the least reactive metal and they are often called the noble metals they can be found in pure form in nature some are used by ancient people to make jewellery and coins.
 Source: slideplayer.com
Source: slideplayer.com
The extraction of the least reactive metals. Platinum is the least reactive metal and exhibits remarkable resistance to corrosion even at high temperature making it extremely valuable for industrial use. It was used to make decorative items such as gates. Most native specimens contain traces of iron and it is likely to be found associated with other elements such as gold copper and nickel in alloyed form or laced with other rare metals. Least reactive metals usually occur in form of free metal.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Examples are zinc iron and lead. It is extremely rare to find platinum in pure form. Noble metals include copper palladium silver platinum and gold. Gold silver titanium palladium platinum. What is the stability of the more reactive metals.
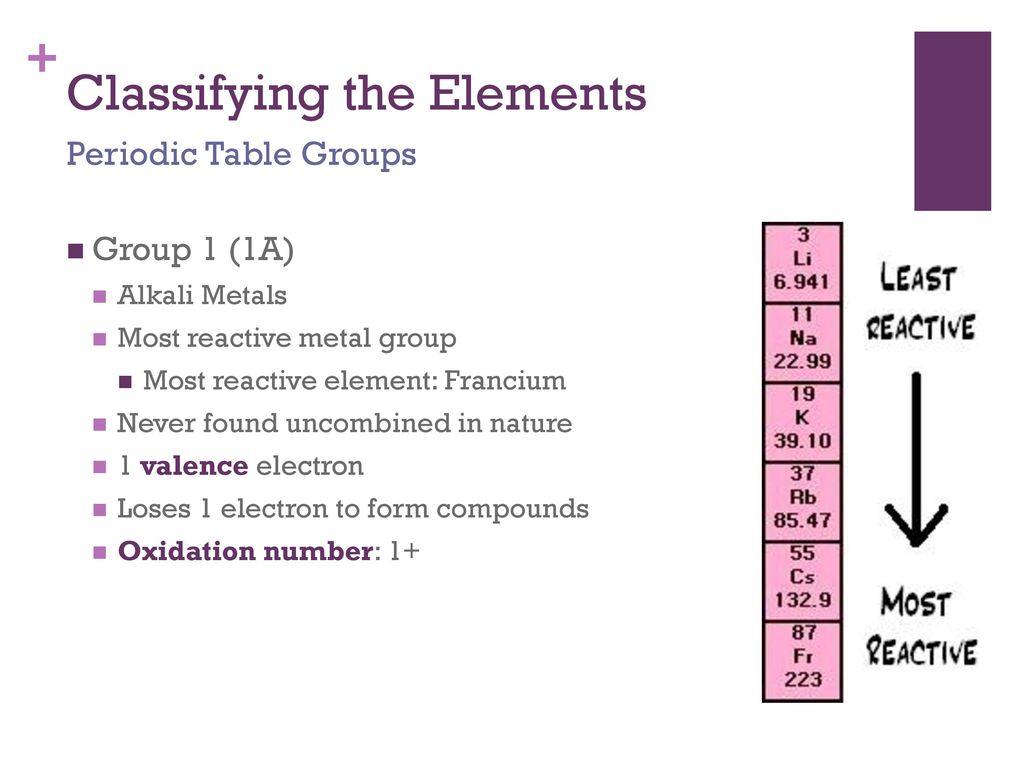 Source: slideplayer.com
Source: slideplayer.com
Examples are gold silver and copper. Gold platinum iridium and rhodium. It is extremely rare to find platinum in pure form. Metals from middle order of reactivity series are found in form of oxides sulphides and carbonates. Noble metals are found as pure metals because they are nonreactive and don t combine with other elements to form compounds.
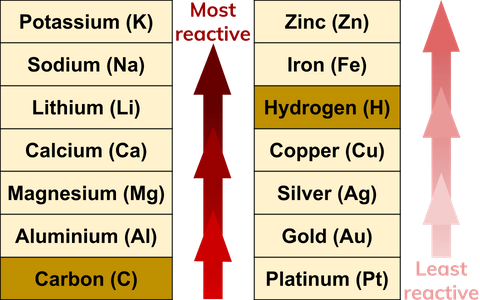 Source: senecalearning.com
Source: senecalearning.com
The more stable the compound the more difficult it is to extract the metals for it. Gold silver titanium palladium platinum. The more reactive the more stable it is as a compound. Examples are gold silver and copper. They are cleaned through various chemical reactions.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what are the least reactive metals found in pure form by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.