What is the polarity of water molecules
What Is The Polarity Of Water Molecules. The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water h 2 o is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. The dipoles do not cancel out resulting in a net dipole.
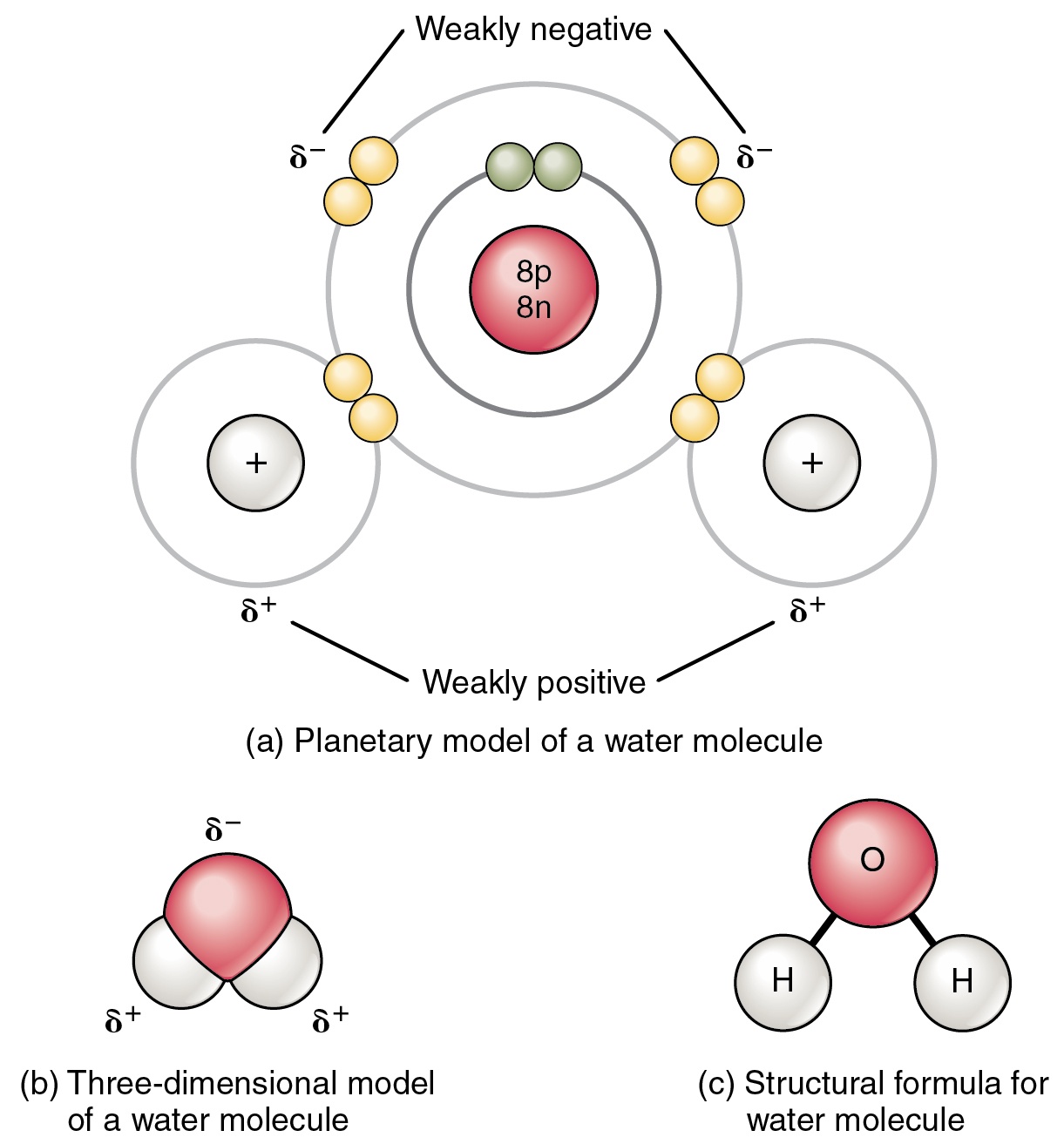 Water Molecular Structure Bonding Expii From expii.com
Water Molecular Structure Bonding Expii From expii.com
The dipoles do not cancel out resulting in a net dipole. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Under certain conditions water also forms a supercritical fluid. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. According to the state of utah division of water resources a water molecule is polar because the oxygen atom at the top of the molecule has a more negative charge while the bottom of the molecule where the hydrogen atoms are found has a more positive charge.
Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
We can tell that some molecules are clearly polar or nonpolar. This is an example of polar covalent chemical bonding. We can tell that some molecules are clearly polar or nonpolar. One of water s important properties is that it is composed of polar molecules. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
 Source: newwatermodel.blogspot.com
Source: newwatermodel.blogspot.com
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Under certain conditions water also forms a supercritical fluid. Water is polar because all of the electrons are bunched up on the oxygen side of a water molecule while fewer electrons are on the hydrogen side of the molecule.
 Source: sciencenotes.org
Source: sciencenotes.org
The polarity of water molecules can explain why certain characteristics of water exist such as its ability to dissolve other substances its density and the strong bonds that hold the molecules together. The dipoles do not cancel out resulting in a net dipole. The molecule has an overall neutral charge because the two charges cancel each other. We can tell that some molecules are clearly polar or nonpolar. In methane all of the electrons are equally distributed.
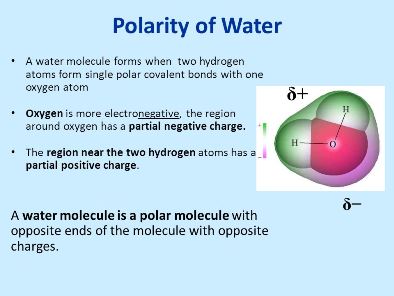 Source: masteringbiologyquiz.com
Source: masteringbiologyquiz.com
The polarity of water is one of the most important things in order to know how it would react with another substance. Water h 2 o is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. Water is polar because all of the electrons are bunched up on the oxygen side of a water molecule while fewer electrons are on the hydrogen side of the molecule. A polar molecule has a net dipole as a result of the opposing charges i e. Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
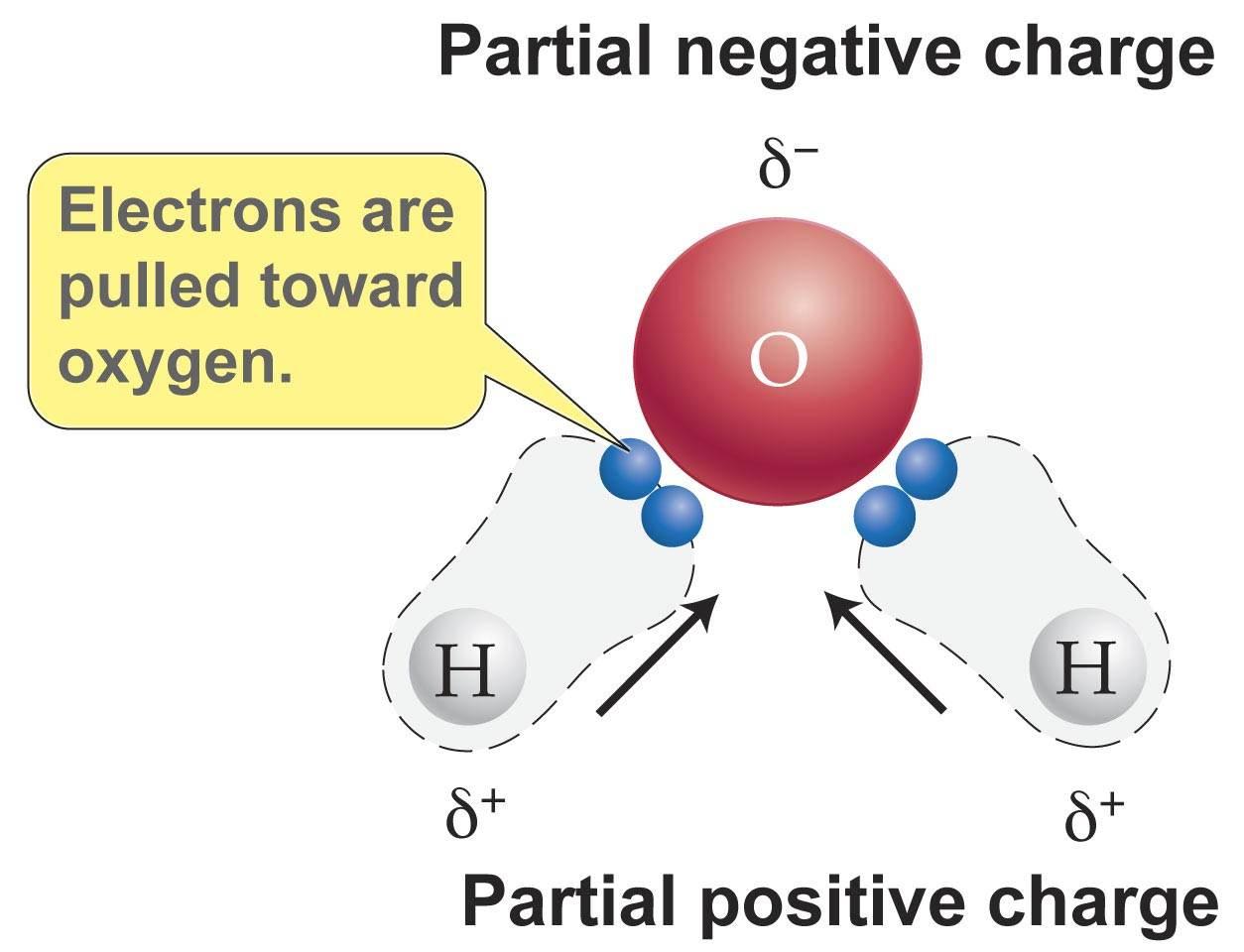 Source: socratic.org
Source: socratic.org
We can tell that some molecules are clearly polar or nonpolar. The polarity of water molecules can explain why certain characteristics of water exist such as its ability to dissolve other substances its density and the strong bonds that hold the molecules together. The polarity of water is one of the most important things in order to know how it would react with another substance. The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. Water is polar because all of the electrons are bunched up on the oxygen side of a water molecule while fewer electrons are on the hydrogen side of the molecule.
 Source: biology.arizona.edu
Source: biology.arizona.edu
The molecule has an overall neutral charge because the two charges cancel each other. The two main classes of molecules are polar molecules and nonpolar molecules. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. In methane all of the electrons are equally distributed. The geometry in turn is due to oxygen s two lone pairs.
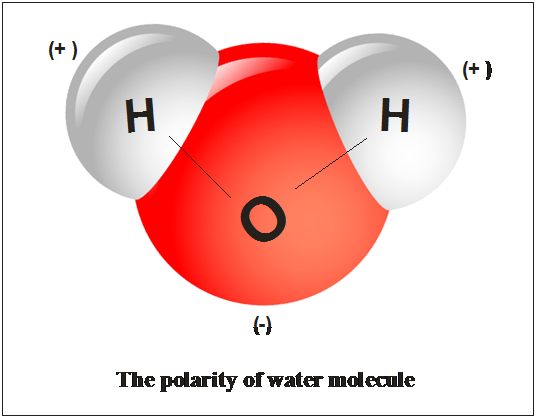 Source: chegg.com
Source: chegg.com
The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. A polar molecule has a net dipole as a result of the opposing charges i e. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This causes on end of the molecule to be negative while the other is positive.
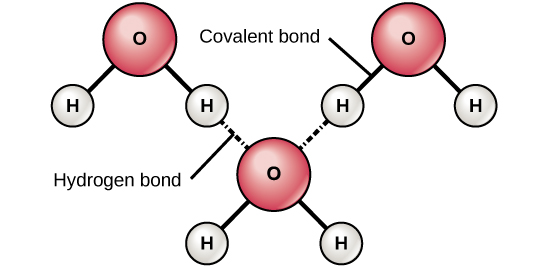 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
In methane all of the electrons are equally distributed. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Polarity of a water molecule water h 2 o is polar because of the bent shape of the molecule. Having partial positive and partial negative charges from polar bonds arranged asymmetrically. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
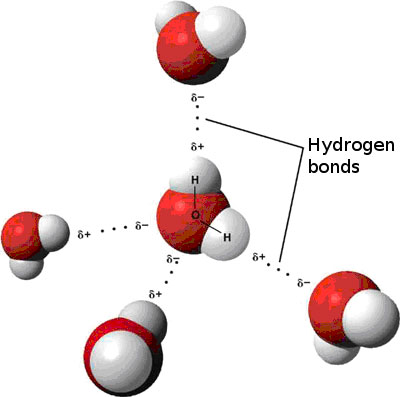 Source: sciencepartners.info
Source: sciencepartners.info
The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. This is an example of polar covalent chemical bonding. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen contributing to water s properties of attraction. The molecule has an overall neutral charge because the two charges cancel each other. The dipoles do not cancel out resulting in a net dipole.
 Source: expii.com
Source: expii.com
A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The polarity of water molecules can explain why certain characteristics of water exist such as its ability to dissolve other substances its density and the strong bonds that hold the molecules together. These characteristics not only maintain life through biochemical processes but also create the hospitable environments that sustain life. The geometry in turn is due to oxygen s two lone pairs. According to the state of utah division of water resources a water molecule is polar because the oxygen atom at the top of the molecule has a more negative charge while the bottom of the molecule where the hydrogen atoms are found has a more positive charge.
 Source: m.youtube.com
Source: m.youtube.com
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water is polar because all of the electrons are bunched up on the oxygen side of a water molecule while fewer electrons are on the hydrogen side of the molecule. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. In methane all of the electrons are equally distributed. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons.
 Source: khanacademy.org
Source: khanacademy.org
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The polarity of water is one of the most important things in order to know how it would react with another substance. The two main classes of molecules are polar molecules and nonpolar molecules.
 Source: usgs.gov
Source: usgs.gov
Water is polar because all of the electrons are bunched up on the oxygen side of a water molecule while fewer electrons are on the hydrogen side of the molecule. Water h 2 o is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. Water h 2 o is a polar molecule and a polar solvent. In methane all of the electrons are equally distributed. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
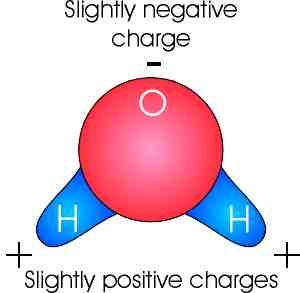 Source: marinebiology.org
Source: marinebiology.org
The polarity of water molecules can explain why certain characteristics of water exist such as its ability to dissolve other substances its density and the strong bonds that hold the molecules together. The two main classes of molecules are polar molecules and nonpolar molecules. Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. These characteristics not only maintain life through biochemical processes but also create the hospitable environments that sustain life.
 Source: sciencenotes.org
Source: sciencenotes.org
Having partial positive and partial negative charges from polar bonds arranged asymmetrically. Water h 2 o is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. Polarity of a water molecule water h 2 o is polar because of the bent shape of the molecule. A polar molecule has a net dipole as a result of the opposing charges i e. Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
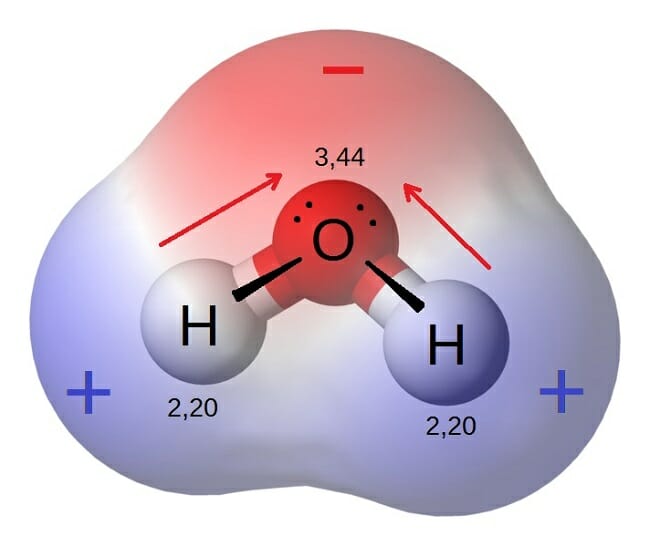 Source: biologydictionary.net
Source: biologydictionary.net
A polar molecule has a net dipole as a result of the opposing charges i e. The polarity of water is one of the most important things in order to know how it would react with another substance. We can tell that some molecules are clearly polar or nonpolar. Having partial positive and partial negative charges from polar bonds arranged asymmetrically. Polarity of a water molecule water h 2 o is polar because of the bent shape of the molecule.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the polarity of water molecules by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







