What metals rust
What Metals Rust. Rust is the orange brown discoloration that builds up on metal. All metals will rust but at differing rates. Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. The most familiar form of rust is the reddish coating that forms flakes on iron and steel fe 2 o 3 but rust also comes in other colors including yellow brown orange and even green.
 Scrapsimple Tools Styles Aged Metals Iii Rust 8501 Biggie From store.scrapgirls.com
Scrapsimple Tools Styles Aged Metals Iii Rust 8501 Biggie From store.scrapgirls.com
Rust is a form of iron oxide. The different colors reflect various chemical compositions of rust. Rust forms when iron undergoes the process of oxidation but not all oxidation forms rust. But many stainless steel alloys also contain a high percentage of chromium at least 18 percent which is even more reactive than iron. Stainless steel types such as 304 or 316 are a mix of elements and most contain some amount of iron which easily oxidizes to form rust. Corrosion occurs when metals begin to deteriorate as a result of chemical reactions between a particular metal and its surrounding environment.
Rate of deterioration will depend on the environmental conditions.
Metals that don t contain iron like aluminum and titanium will not rust although they will oxidize. While rust only appears on iron and iron based alloys corrosion can occur on almost every metal. Rust can affect iron and its alloys including steel. This compound contains water molecules so we call it a hydrated compound. Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. Whenever you have iron water and oxygen together you get rust.
 Source: tampasteel.com
Source: tampasteel.com
Rust can affect iron and its alloys including steel. Gold and platinum oxides are very rare because not only will it take many years for these metals to rust but the amount will be very insignificant. Metals that don t contain iron like aluminum and titanium will not rust although they will oxidize. Usually rust occurs when iron based metals come into contact with water saltwater acidic substances etc. Corrosion occurs when metals begin to deteriorate as a result of chemical reactions between a particular metal and its surrounding environment.
 Source: xapps.xyleminc.com
Source: xapps.xyleminc.com
The most commonly used metal that contains iron is steel. Rusting is an electrochemical process that reduces iron containing metals into their natural unrefined states. The most familiar form of rust is the reddish coating that forms flakes on iron and steel fe 2 o 3 but rust also comes in other colors including yellow brown orange and even green. Even gold platinum and silver will rust but the process is very slow and produces various colors of tarnish. All metals will rust but at differing rates.
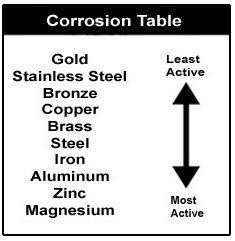
Rust can affect iron and its alloys including steel. Rust can affect iron and its alloys including steel. Rate of deterioration will depend on the environmental conditions. Rust is a form of iron oxide. All metals will rust but at differing rates.
 Source: thoughtco.com
Source: thoughtco.com
What causes metal to rust. Whenever you have iron water and oxygen together you get rust. Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. Corrosion on the other hand is used as a broad term to describe the destruction of metals due to chemical or electrochemical reactions. What causes metal to rust.
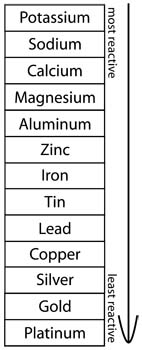 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. The most familiar form of rust is the reddish coating that forms flakes on iron and steel fe 2 o 3 but rust also comes in other colors including yellow brown orange and even green. Whenever you have iron water and oxygen together you get rust. Ferrous metals those made of or from iron are the only metals that can rust but all base metals can corrode. Usually rust occurs when iron based metals come into contact with water saltwater acidic substances etc.
 Source: store.scrapgirls.com
Source: store.scrapgirls.com
Rust can affect iron and its alloys including steel. What causes metal to rust. While rust only appears on iron and iron based alloys corrosion can occur on almost every metal. Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. Usually rust occurs when iron based metals come into contact with water saltwater acidic substances etc.
 Source: techsteel.net
Source: techsteel.net
Both oxygen gas and water must be present for the iron to rust. Rust is a form of iron oxide. For starters only metal that contains iron will rust. Rust is also seen as tarnish on some metals. Usually rust occurs when iron based metals come into contact with water saltwater acidic substances etc.
 Source: amazon.com
Source: amazon.com
This compound contains water molecules so we call it a hydrated compound. What causes metal to rust. Both oxygen gas and water must be present for the iron to rust. The most familiar form of rust is the reddish coating that forms flakes on iron and steel fe 2 o 3 but rust also comes in other colors including yellow brown orange and even green. Corrosion occurs when metals begin to deteriorate as a result of chemical reactions between a particular metal and its surrounding environment.
 Source: materialsperformance.com
Source: materialsperformance.com
The different colors reflect various chemical compositions of rust. Whenever you have iron water and oxygen together you get rust. For starters only metal that contains iron will rust. Metals that don t contain iron like aluminum and titanium will not rust although they will oxidize. Rust is the orange brown discoloration that builds up on metal.
 Source: manvillerecycling.com
Source: manvillerecycling.com
This compound contains water molecules so we call it a hydrated compound. It occurs when iron combines with the oxygen in the air causing it to corrode. The different colors reflect various chemical compositions of rust. Metals that don t contain iron like aluminum and titanium will not rust although they will oxidize. Rust occurs when metals containing iron react with the oxygen in the air or in water and form a compound called iron iii oxide ferric oxide.
 Source: pinterest.com
Source: pinterest.com
What causes metal to rust. Whenever you have iron water and oxygen together you get rust. This compound contains water molecules so we call it a hydrated compound. Metals that don t contain iron like aluminum and titanium will not rust although they will oxidize. The most commonly used metal that contains iron is steel.
 Source: tampasteel.com
Source: tampasteel.com
This compound contains water molecules so we call it a hydrated compound. The different colors reflect various chemical compositions of rust. It occurs when iron combines with the oxygen in the air causing it to corrode. But many stainless steel alloys also contain a high percentage of chromium at least 18 percent which is even more reactive than iron. Rust is the orange brown discoloration that builds up on metal.
 Source: thoughtco.com
Source: thoughtco.com
Rust can affect iron and its alloys including steel. Rust is the orange brown discoloration that builds up on metal. Whenever you have iron water and oxygen together you get rust. But many stainless steel alloys also contain a high percentage of chromium at least 18 percent which is even more reactive than iron. Rust is a form of iron oxide.
 Source: docbrown.info
Source: docbrown.info
Rust is the common name for iron oxide. Rust is the common name for iron oxide. Rust can affect iron and its alloys including steel. Usually rust occurs when iron based metals come into contact with water saltwater acidic substances etc. Rust occurs when metals containing iron react with the oxygen in the air or in water and form a compound called iron iii oxide ferric oxide.
 Source: scienceabc.com
Source: scienceabc.com
Rust is also seen as tarnish on some metals. Rust forms when iron undergoes the process of oxidation but not all oxidation forms rust. Rust commonly referred to as oxidation occurs when iron or metal alloys that contain iron such as steel are exposed to oxygen and water for a long period of time. What causes metal to rust. Stainless steel types such as 304 or 316 are a mix of elements and most contain some amount of iron which easily oxidizes to form rust.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what metals rust by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






