Where are metals in the periodic table
Where Are Metals In The Periodic Table. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Nonmetals are located on the righthand side of the periodic table. The three broad categories of elements are metals nonmetals and metalloids. All of the metals are grouped together on the left side of the periodic table.
 Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals From theconversation.com
Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals From theconversation.com
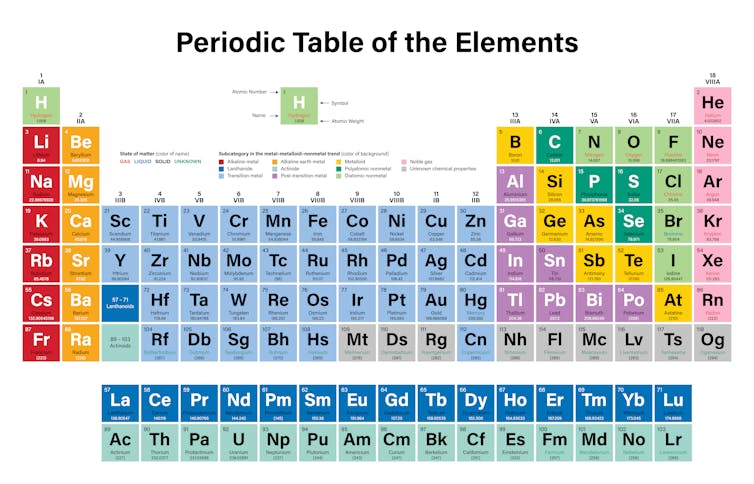
The columns of the periodic table are called groups. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. The remaining elements are either metalloids b si ge as sb and te being commonly recognised as such or nonmetals. This periodic table groups elements according to type. All elements in a group share the same number of valence electrons. Metalloids have properties of both metals and nonmetals.
Most elements can be considered metals.
The number of each element corresponds to the number of protons in its nucleus which is the same as the number of electrons orbiting that nucleus. The metalloids separate the metals and nonmetals on a periodic table. The number of each element corresponds to the number of protons in its nucleus which is the same as the number of electrons orbiting that nucleus. Metalloids have properties of both metals and nonmetals. The three broad categories of elements are metals nonmetals and metalloids. All elements in a group share the same number of valence electrons.
 Source: pinterest.com
Source: pinterest.com
In chemistry the elements which are usually considered to be metals under ordinary conditions are shown in yellow on the periodic table below. Interactive periodic table with up to date element property data collected from authoritative sources. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. This periodic table groups elements according to type. In chemistry the elements which are usually considered to be metals under ordinary conditions are shown in yellow on the periodic table below.
Source: igcse-chemistry-2017.blogspot.com
Most elements can be considered metals. Nonmetals are located on the righthand side of the periodic table. The metalloids separate the metals and nonmetals on a periodic table. The three broad categories of elements are metals nonmetals and metalloids. Most elements can be considered metals.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. Most elements can be considered metals. The metalloids separate the metals and nonmetals on a periodic table. The three broad categories of elements are metals nonmetals and metalloids. Metalloids have properties of both metals and nonmetals.
 Source: theconversation.com
Source: theconversation.com
Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. In chemistry the elements which are usually considered to be metals under ordinary conditions are shown in yellow on the periodic table below. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. Interactive periodic table with up to date element property data collected from authoritative sources.
 Source: researchgate.net
Source: researchgate.net
The periodic table also known as the periodic table of elements arranges the chemical elements such as hydrogen silicon iron and uranium according to their recurring properties. The columns of the periodic table are called groups. The remaining elements are either metalloids b si ge as sb and te being commonly recognised as such or nonmetals. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.
Source: researchgate.net
Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. Metalloids have properties of both metals and nonmetals. Most elements are metals. The number of each element corresponds to the number of protons in its nucleus which is the same as the number of electrons orbiting that nucleus. The remaining elements are either metalloids b si ge as sb and te being commonly recognised as such or nonmetals.
 Source: socratic.org
Source: socratic.org
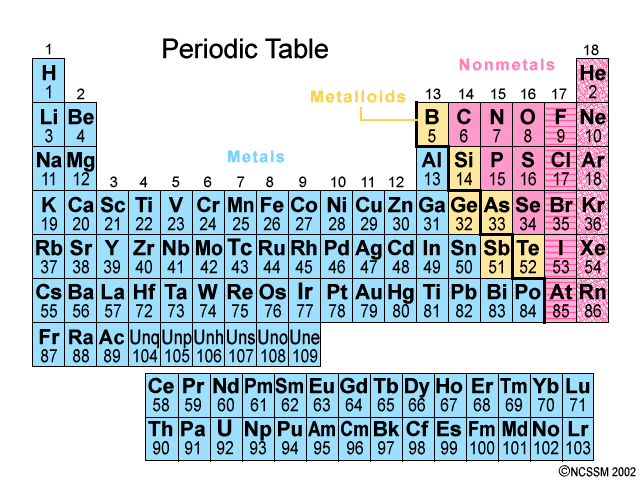
This periodic table groups elements according to type. They are grouped together in the middle to the left hand side of the periodic table. In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84. This periodic table groups elements according to type. The remaining elements are either metalloids b si ge as sb and te being commonly recognised as such or nonmetals.
 Source: breakingatom.com
Source: breakingatom.com
Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. The metalloids separate the metals and nonmetals on a periodic table. Most elements can be considered metals. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game.
 Source: thoughtco.com
Source: thoughtco.com
The metals share several common properties including. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. The three broad categories of elements are metals nonmetals and metalloids. The line begins at boron b and extends down to polonium po. Metal blue nonmetal yellow or metalloid red.
 Source: sciencenotes.org
Source: sciencenotes.org
The number of each element corresponds to the number of protons in its nucleus which is the same as the number of electrons orbiting that nucleus. The remaining elements are either metalloids b si ge as sb and te being commonly recognised as such or nonmetals. Metalloids have properties of both metals and nonmetals. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals. In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84.
 Source: angelo.edu
Source: angelo.edu
The periodic table also known as the periodic table of elements arranges the chemical elements such as hydrogen silicon iron and uranium according to their recurring properties. In chemistry the elements which are usually considered to be metals under ordinary conditions are shown in yellow on the periodic table below. In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84. The line begins at boron b and extends down to polonium po. All elements in a group share the same number of valence electrons.
Source: quora.com
Interactive periodic table with up to date element property data collected from authoritative sources. Also many periodic tables have a stair step line on the table identifying the element groups. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Nonmetals are located on the righthand side of the periodic table.
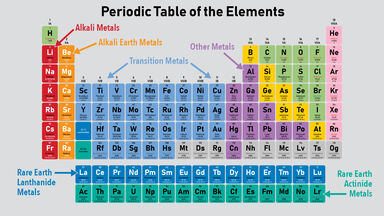 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Metal blue nonmetal yellow or metalloid red. This periodic table groups elements according to type. The metals share several common properties including. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Nonmetals are located on the righthand side of the periodic table.
 Source: britannica.com
Source: britannica.com
Also many periodic tables have a stair step line on the table identifying the element groups. The columns of the periodic table are called groups. If you look at the periodic table you will find that the metal elements are located between atomic number 5 boron b all the way to atomic number 84 polonium po. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. Elements to the left of the line are considered metals.
 Source: youtube.com
Source: youtube.com
Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Most elements can be considered metals. Metals reside on the left side of the table while non metals reside on the right. This periodic table groups elements according to type. The metalloids separate the metals and nonmetals on a periodic table.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title where are metals in the periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.