Where are the most reactive metals on the periodic table
Where Are The Most Reactive Metals On The Periodic Table. They are never found uncombined and only exist as compounds in nature. It is highly acquiescent and readily gives up the single electron in its valence shell to attain stability making it highly reactive. 2 na s 2 h 2 o l 2 naoh aq h 2 g. Cesium reacts explosively with water though it is predicted francium would react even more vigorously.
 What Is The Most Reactive Metal On The Periodic Table Socratic From socratic.org
What Is The Most Reactive Metal On The Periodic Table Socratic From socratic.org
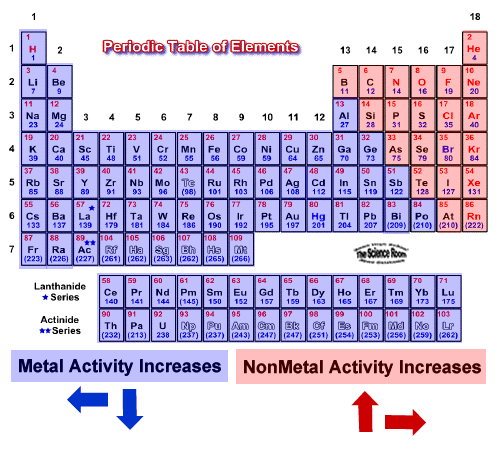
The most reactive metals are located at the bottom left corner of periodic table. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. See full answer below. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. They are never found uncombined and only exist as compounds in nature.
The most reactive metal in the periodic table is francium.
The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. As we move down the group in this column metallic character increase. Updated october 08 2019 the most reactive metal on the periodic table is francium. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. Cesium reacts explosively with water though it is predicted francium would react even more vigorously.
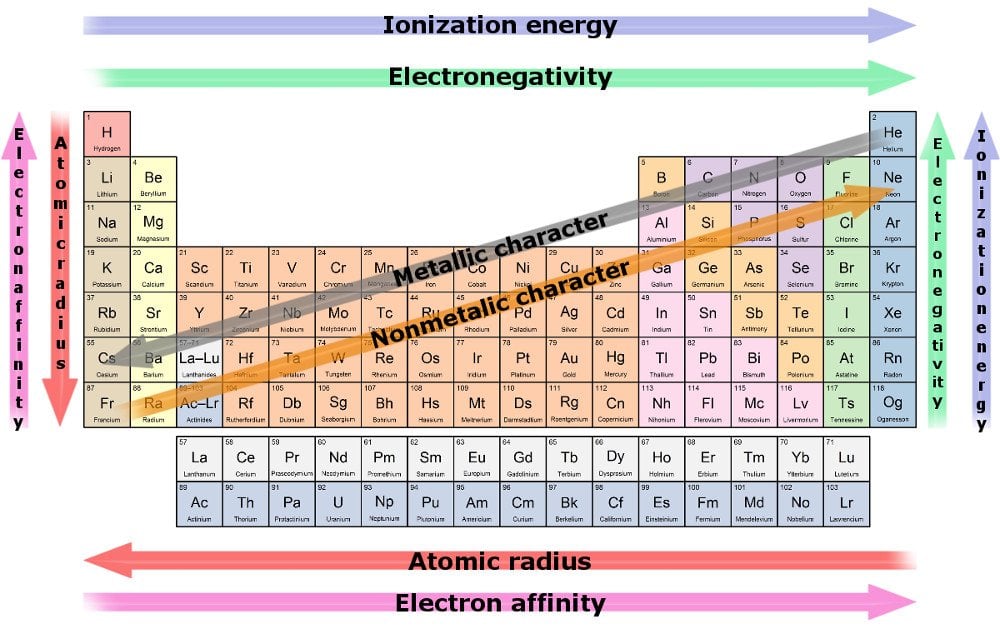 Source: scienceabc.com
Source: scienceabc.com
Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. The most reactive nonmetals are the halogens of group 17 which are located on the far right hand side of the periodic table just before the noble. They are never found uncombined and only exist as compounds in nature. The most reactive metal in the periodic table is francium. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals.
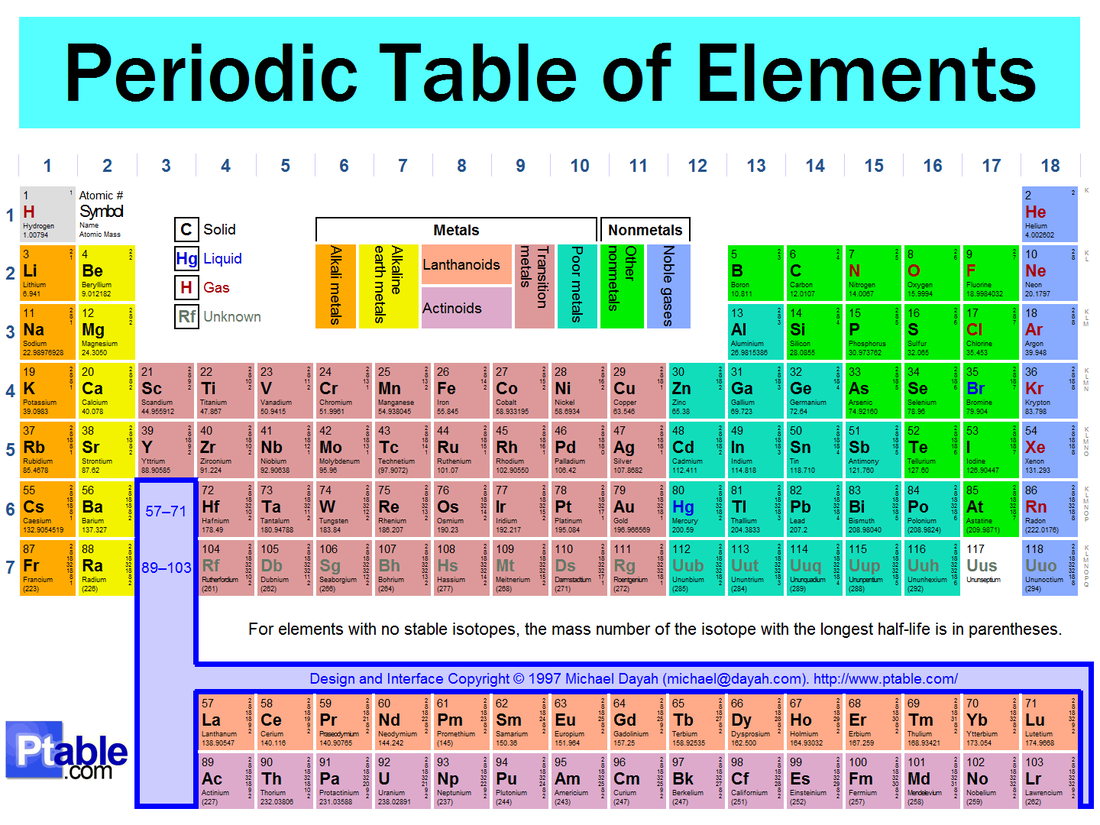 Source: hungergames4chemistry.weebly.com
Source: hungergames4chemistry.weebly.com
Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. It is highly acquiescent and readily gives up the single electron in its valence shell to attain stability making it highly reactive. Group 1 is known as the alkali metals. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. The most reactive metals are located at the bottom left corner of periodic table.
 Source: pinterest.com
Source: pinterest.com
See full answer below. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Updated october 08 2019 the most reactive metal on the periodic table is francium. 2 na s 2 h 2 o l 2 naoh aq h 2 g. They are never found uncombined and only exist as compounds in nature.
 Source: toppr.com
Source: toppr.com
These metals are reactive in the sense that they can react with water very easily. Look at the configurations of all the elements down group 1. The most reactive nonmetals are the halogens of group 17 which are located on the far right hand side of the periodic table just before the noble. They are the most reactive metals in the periodic table. Examine your periodic table.
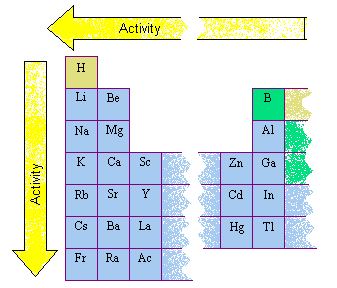 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
They are never found uncombined and only exist as compounds in nature. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. The most reactive nonmetals are the halogens of group 17 which are located on the far right hand side of the periodic table just before the noble. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. Updated october 08 2019 the most reactive metal on the periodic table is francium.
 Source: socratic.org
Source: socratic.org
Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. It is highly acquiescent and readily gives up the single electron in its valence shell to attain stability making it highly reactive. Group 1 is known as the alkali metals. Examine your periodic table. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals.
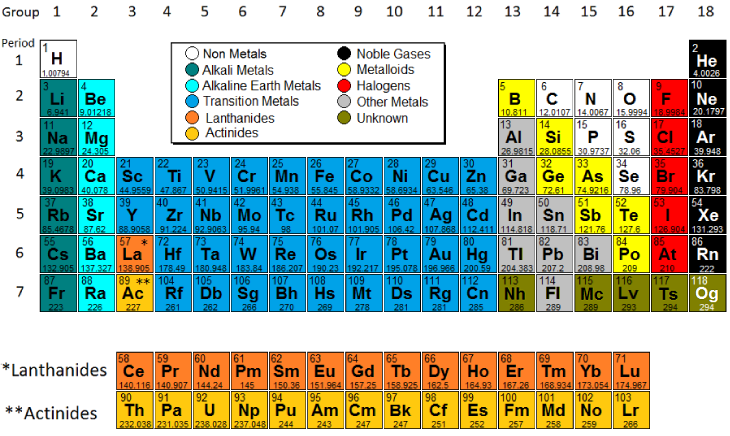 Source: modelscience.com
Source: modelscience.com
As we move down the group in this column metallic character increase. The most reactive metal in the periodic table is francium. Examine your periodic table. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
They are never found uncombined and only exist as compounds in nature. 2 na s 2 h 2 o l 2 naoh aq h 2 g. Group 1 is known as the alkali metals. As we move down the group in this column metallic character increase. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. The most reactive nonmetals are the halogens of group 17 which are located on the far right hand side of the periodic table just before the noble. They are never found uncombined and only exist as compounds in nature. As we move down the group in this column metallic character increase.
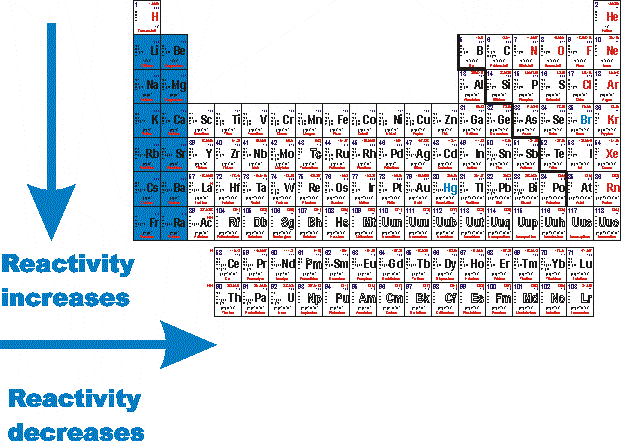 Source: socratic.org
Source: socratic.org
The most reactive metal in the periodic table is francium. The most reactive metal in the periodic table is francium. Pay specific attention to the outermost shell level the last number. 2 na s 2 h 2 o l 2 naoh aq h 2 g. The most reactive metals are located at the bottom left corner of periodic table.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
These metals are highly reactive because they all have only one valence electron. Examples include lithium sodium potassium. Francium however is a laboratory produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive. These metals are highly reactive because they all have only one valence electron.
Source: quora.com
As we move down the group in this column metallic character increase. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Cesium reacts explosively with water though it is predicted francium would react even more vigorously. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. The most reactive metals are located at the bottom left corner of periodic table.
 Source: slideplayer.com
Source: slideplayer.com
The most reactive metals are located at the bottom left corner of periodic table. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Group 1 is known as the alkali metals. Examine your periodic table. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
Source: quora.com
The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. Examples include lithium sodium potassium. It is highly acquiescent and readily gives up the single electron in its valence shell to attain stability making it highly reactive. Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
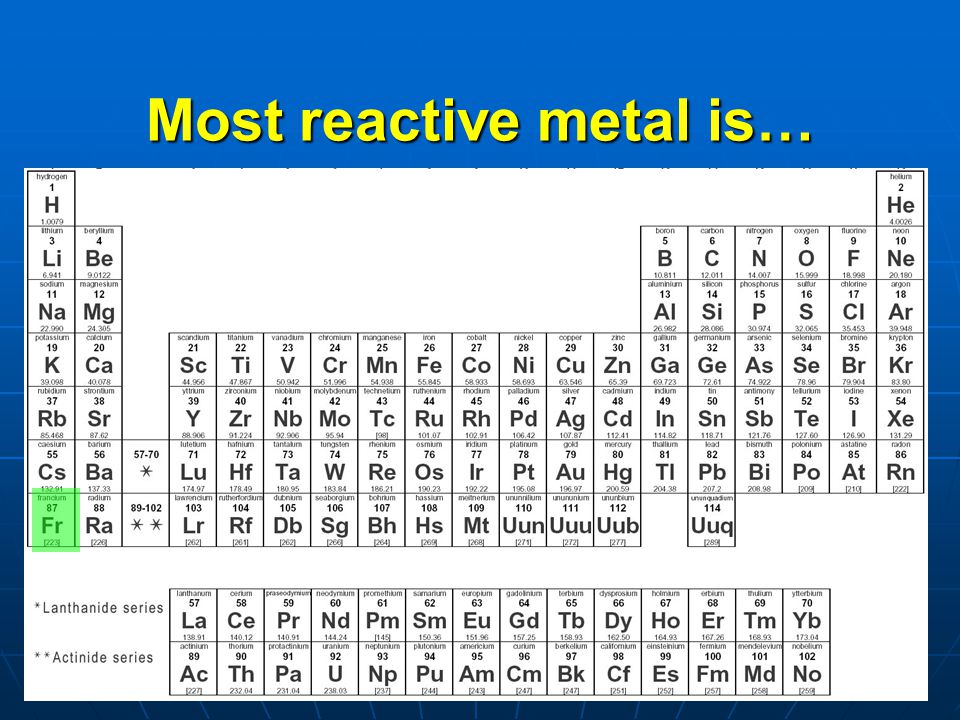 Source: periodictable.me
Source: periodictable.me
Pay specific attention to the outermost shell level the last number. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. These metals are reactive in the sense that they can react with water very easily. The most reactive metal in the periodic table is francium. It is highly acquiescent and readily gives up the single electron in its valence shell to attain stability making it highly reactive.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title where are the most reactive metals on the periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.