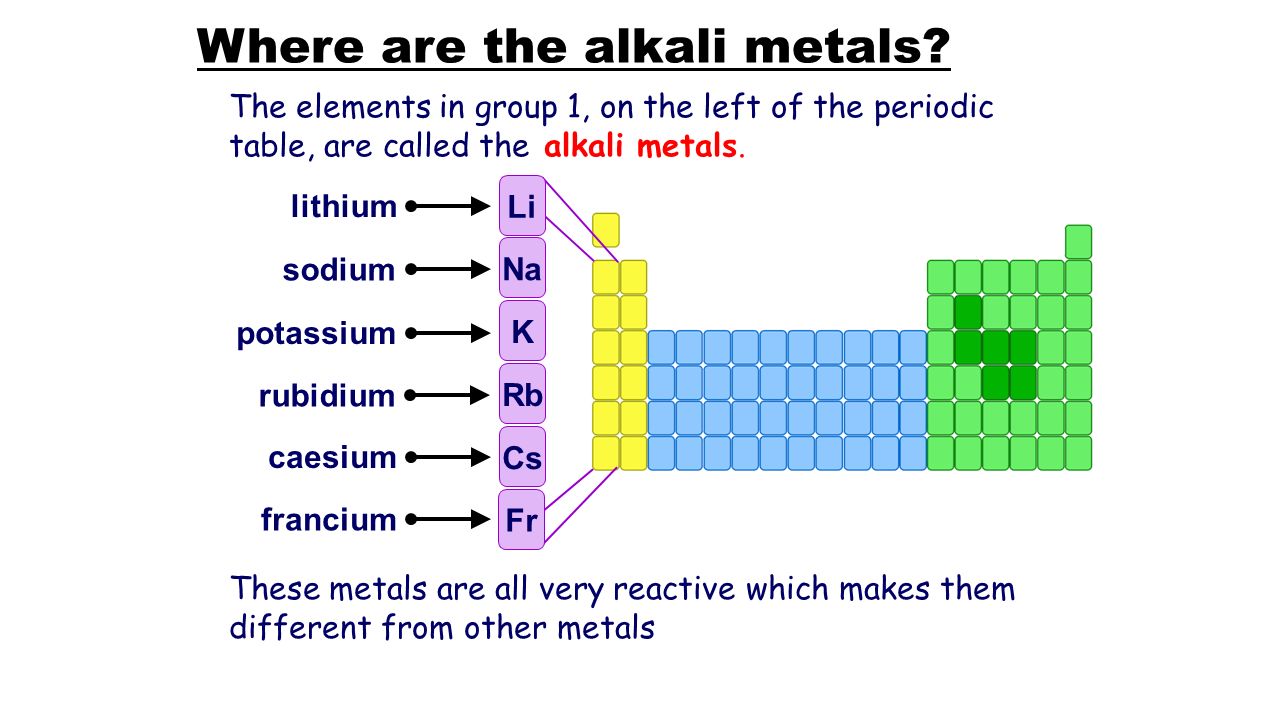
Which alkali metal is most reactive
Which Alkali Metal Is Most Reactive. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron. These metals are characterized by their soft texture and silvery color. Alkali metals are more reactive generally than alkaline earth metals since alkali metals only has 1 valence electron.
 The Periodic Table Alkali Metals Alkali Metals Most Reactive Metal Group On Periodic Table Most Reactive Metal Ppt Download From slideplayer.com
The Periodic Table Alkali Metals Alkali Metals Most Reactive Metal Group On Periodic Table Most Reactive Metal Ppt Download From slideplayer.com
Because the reactivity of alkali metals they tend to combine readily and explosively with metals particularly with alkali metals. These metals are characterized by their soft texture and silvery color. All the alkali metals react with water with the heavier alkali metals reacting more vigorously than the lighter ones. Among the alkali metals cesium is the most reactive because. Aa alkaline batteries line up in rows. For example any of the alkalis and fluorine which is very electronegative and wants 1 more electron would be very dangerous because it would complete.
The alkali metals readily loses this electron so that they can achieve.
Among the alkali metals cesium is the most reactive because. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of 1. Alkali metals are among the most reactive metals. These are made with lithium one of the alkali metals on the periodic tables. Among the alkali metals cesium is the most reactive because.
 Source: quora.com
Source: quora.com
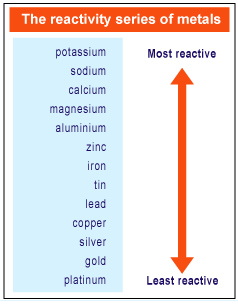
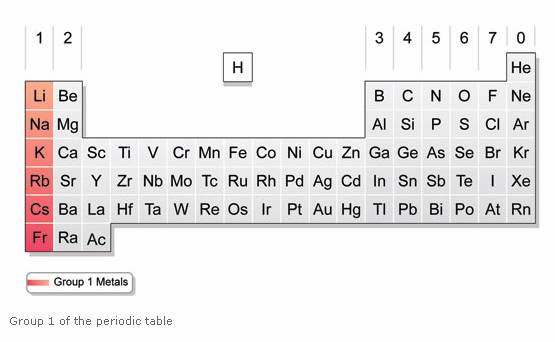
The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. These metals are characterized by their soft texture and silvery color. Because the reactivity of alkali metals they tend to combine readily and explosively with metals particularly with alkali metals. Alkali metals are more reactive generally than alkaline earth metals since alkali metals only has 1 valence electron.
 Source: slideplayer.com
Source: slideplayer.com
These metals are characterized by their soft texture and silvery color. Fluorine is one of the most reactive elements in existence attacking. Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron. The alkali metals readily loses this electron so that they can achieve. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive.
 Source: sites.google.com
Source: sites.google.com
Caesium the fifth alkali metal is the most reactive of all the metals. Fluorine is one of the most reactive elements in existence attacking. This is due in part to their larger atomic radii and low ionization energies. Caesium the fifth alkali metal is the most reactive of all the metals. Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron.
 Source: completeigcsechemistry.blogspot.com
Source: completeigcsechemistry.blogspot.com
Because the reactivity of alkali metals they tend to combine readily and explosively with metals particularly with alkali metals. These are made with lithium one of the alkali metals on the periodic tables. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive. For example any of the alkalis and fluorine which is very electronegative and wants 1 more electron would be very dangerous because it would complete. Nov 04 2007 what alkali metal is the most reactive element and are the most reactive and lithium is the least.
 Source: docbrown.info
Source: docbrown.info
Caesium the fifth alkali metal is the most reactive of all the metals. Because the reactivity of alkali metals they tend to combine readily and explosively with metals particularly with alkali metals. This is due in part to their larger atomic radii and low ionization energies. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Alkali metals are among the most reactive metals.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Among the alkali metals cesium is the most reactive because. They tend to donate their electrons in reactions and have an oxidation state of 1. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. Among the alkali metals cesium is the most reactive because. Because of the alkali metals electron configuration wants to give its one electron to another substance they re highly reactive.
 Source: slideplayer.com
Source: slideplayer.com
The alkali metals readily loses this electron so that they can achieve. The alkali metals readily loses this electron so that they can achieve. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Alkali metals are more reactive generally than alkaline earth metals since alkali metals only has 1 valence electron. Among the alkali metals cesium is the most reactive because.
Source: isbchem1.pbworks.com
They tend to donate their electrons in reactions and have an oxidation state of 1. This is due in part to their larger atomic radii and low ionization energies. Aa alkaline batteries line up in rows. Alkali metals are more reactive generally than alkaline earth metals since alkali metals only has 1 valence electron. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
 Source: youtube.com
Source: youtube.com
This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of 1. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. These metals are characterized by their soft texture and silvery color.
 Source: boomchemistry.blogspot.com
Source: boomchemistry.blogspot.com
For example any of the alkalis and fluorine which is very electronegative and wants 1 more electron would be very dangerous because it would complete. The alkali metals readily loses this electron so that they can achieve. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Because the reactivity of alkali metals they tend to combine readily and explosively with metals particularly with alkali metals. This is due in part to their larger atomic radii and low ionization energies.
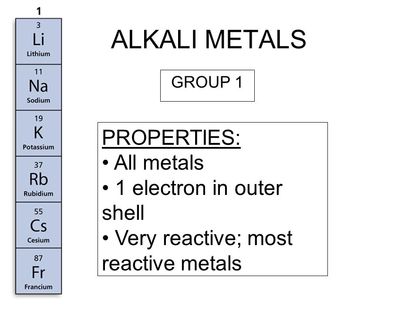 Source: makaylasharanscience2ndperiod.weebly.com
Source: makaylasharanscience2ndperiod.weebly.com
The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Alkali metals are more reactive generally than alkaline earth metals since alkali metals only has 1 valence electron. All the alkali metals react with water with the heavier alkali metals reacting more vigorously than the lighter ones. The alkali metals readily loses this electron so that they can achieve.
 Source: britannica.com
Source: britannica.com
The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Caesium the fifth alkali metal is the most reactive of all the metals. These metals are characterized by their soft texture and silvery color. These are made with lithium one of the alkali metals on the periodic tables. The alkali metals readily loses this electron so that they can achieve.
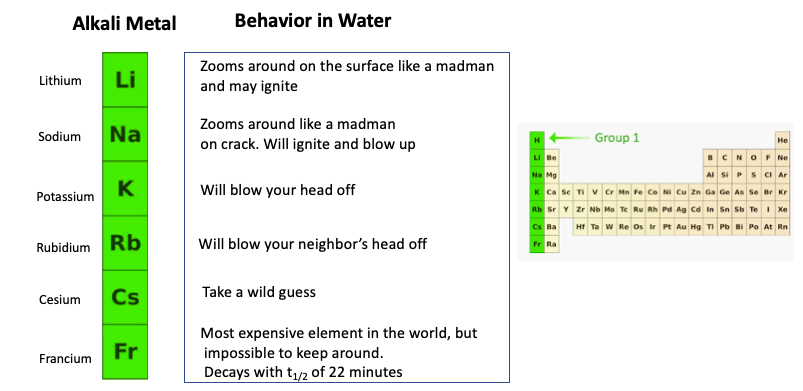 Source: acsh.org
Source: acsh.org
Nov 04 2007 what alkali metal is the most reactive element and are the most reactive and lithium is the least. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. These are made with lithium one of the alkali metals on the periodic tables. All the alkali metals react with water with the heavier alkali metals reacting more vigorously than the lighter ones. Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron.
Source: isbchem1.pbworks.com
For example any of the alkalis and fluorine which is very electronegative and wants 1 more electron would be very dangerous because it would complete. All the alkali metals react with water with the heavier alkali metals reacting more vigorously than the lighter ones. Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron. For example any of the alkalis and fluorine which is very electronegative and wants 1 more electron would be very dangerous because it would complete. Caesium the fifth alkali metal is the most reactive of all the metals.

Among the alkali metals cesium is the most reactive because a its incomplete shell is nearest to the nucleus b it has a single electron. The most reactive metals in the periodic tables are the alkali metals followed by the alkaline earth metals. Alkali metals are among the most reactive metals. Nov 04 2007 what alkali metal is the most reactive element and are the most reactive and lithium is the least. They tend to donate their electrons in reactions and have an oxidation state of 1.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which alkali metal is most reactive by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.