Why are alkali metals so reactive
Why Are Alkali Metals So Reactive. They also have a silver like shine and are great conductors of heat and light. They tend to donate their electrons in reactions and have an oxidation state of 1. These metals are characterized by their soft texture and silvery color. So they readily react with water oxygen which are common constituents in the environment.
 Alkali Metals The Periodic Table From makaylasharanscience2ndperiod.weebly.com
Alkali Metals The Periodic Table From makaylasharanscience2ndperiod.weebly.com
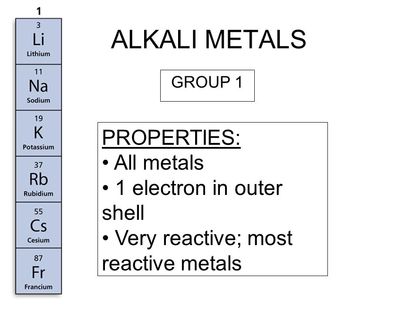
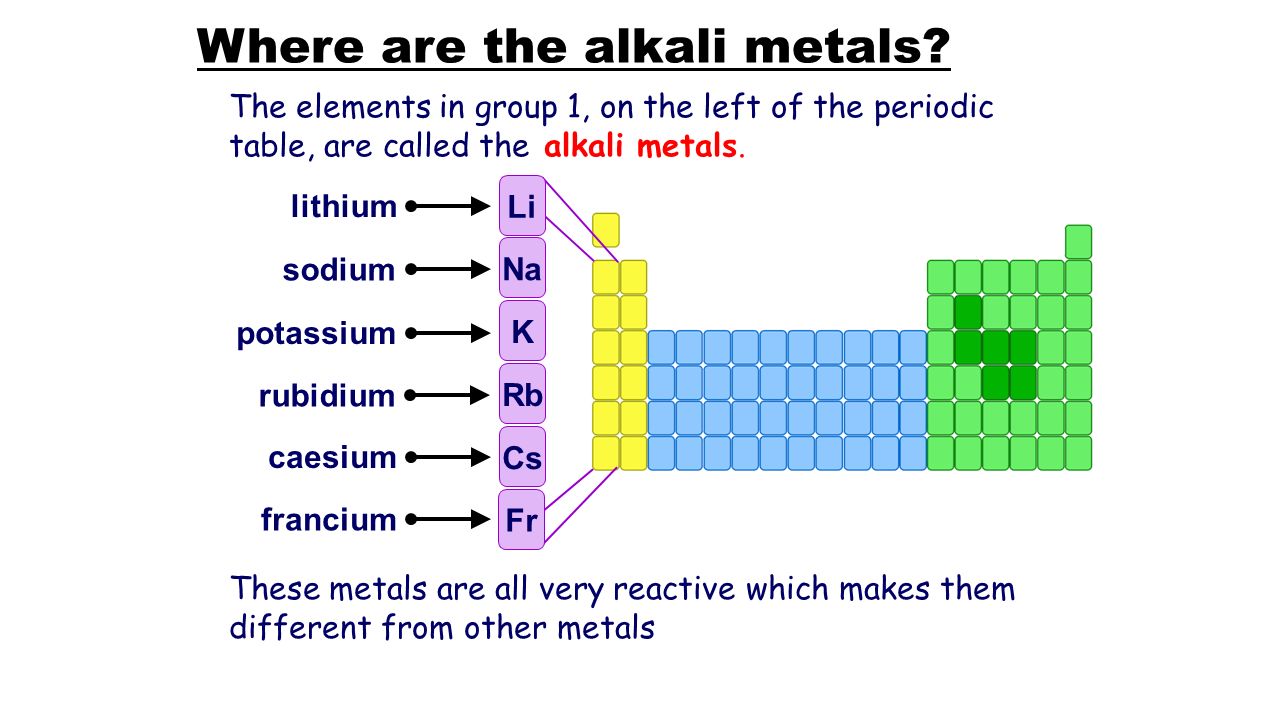
Ionization energy is the energy required to remove one electron from the atom. So we cannot find alkali metals as pure metals. They are trying to loose this electron so they can obtain a noble gas configuration. Alkali metals are among the most reactive metals. Alkali metals are so called because when they react with water they create highly alkaline substances. Why are alkali metals not found in nature.
Alkali metals are reactive because of their ionization energies.
Biological importance of sodium and potassium. Alkali metals are reactive because of their ionization energies. So we cannot find alkali metals as pure metals. The alkali metals are soft metals that are highly reactive with water and oxygen. Biological importance of sodium and potassium. Alkali metals are in group 1 so they have only one electron in their outermost shell.
 Source: youtube.com
Source: youtube.com
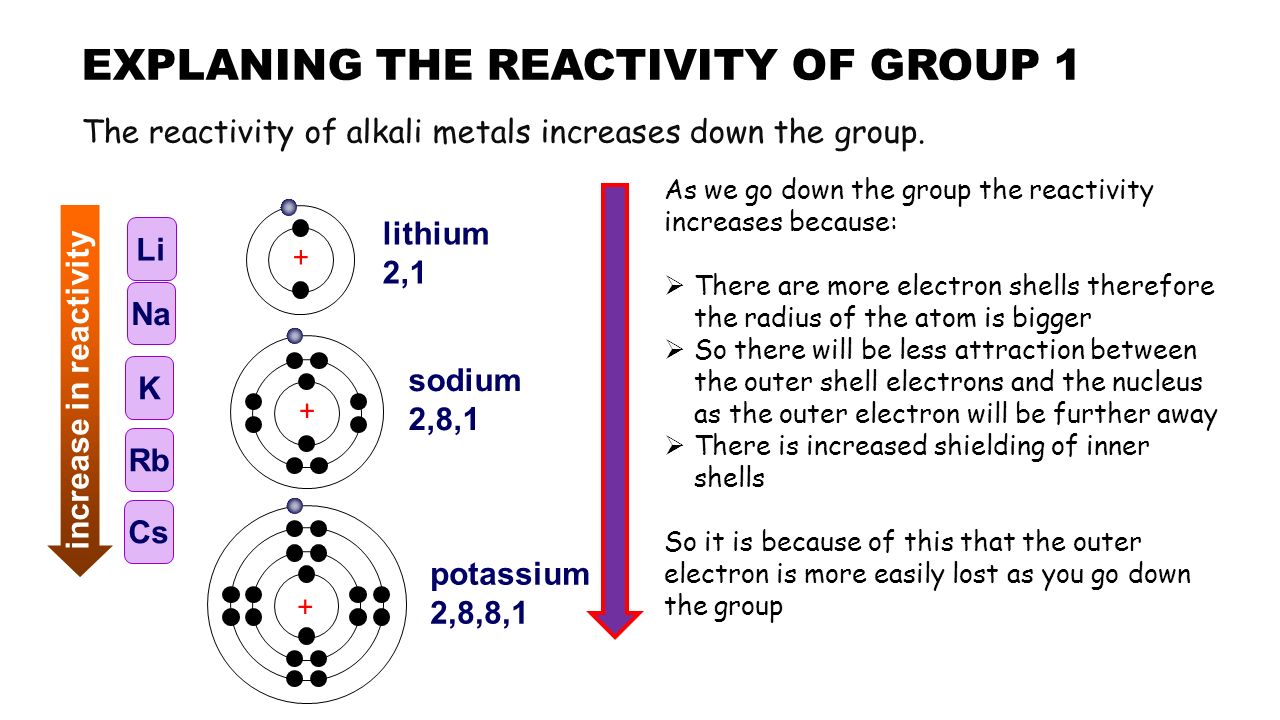
They also have a silver like shine and are great conductors of heat and light. So we cannot find alkali metals as pure metals. As a result of their weak electrostatic attraction metals have low ionisation energies meaning it s easier for them to lose electrons. So they readily react with water oxygen which are common constituents in the environment. They re so soft that you can cut them with a plastic knife.
 Source: slideplayer.com
Source: slideplayer.com
Alkali metals are in group 1 so they have only one electron in their outermost shell. They tend to donate their electrons in reactions and have an oxidation state of 1. They are trying to loose this electron so they can obtain a noble gas configuration. They re so soft that you can cut them with a plastic knife. As a result of their weak electrostatic attraction metals have low ionisation energies meaning it s easier for them to lose electrons.
 Source: breakingatom.com
Source: breakingatom.com
They also have a silver like shine and are great conductors of heat and light. They also have a silver like shine and are great conductors of heat and light. These metals are characterized by their soft texture and silvery color. They re so soft that you can cut them with a plastic knife. Alkali metals are very reactive.
 Source: slideplayer.com
Source: slideplayer.com
When it s easier for a metal to lose electrons that means. They re so soft that you can cut them with a plastic knife. Alkali metals are among the most reactive metals. These metals are characterized by their soft texture and silvery color. As a result of their weak electrostatic attraction metals have low ionisation energies meaning it s easier for them to lose electrons.
 Source: makaylasharanscience2ndperiod.weebly.com
Source: makaylasharanscience2ndperiod.weebly.com
Alkali metals are in group 1 so they have only one electron in their outermost shell. Alkali metals are so called because when they react with water they create highly alkaline substances. They re so soft that you can cut them with a plastic knife. So they readily react with water oxygen which are common constituents in the environment. This is due in part to their larger atomic radii and low ionization energies.
 Source: docbrown.info
Source: docbrown.info
This means that they will react violently to do so as it does not require very much energy to pull away that one electron. Biological importance of sodium and potassium. So they readily react with water oxygen which are common constituents in the environment. This means that they will react violently to do so as it does not require very much energy to pull away that one electron. Alkali metals are in group 1 so they have only one electron in their outermost shell.
Source: isbchem1.pbworks.com
Alkali metals are very reactive. These metals are characterized by their soft texture and silvery color. The alkali metals are soft metals that are highly reactive with water and oxygen. They are trying to loose this electron so they can obtain a noble gas configuration. They also have a silver like shine and are great conductors of heat and light.
 Source: quora.com
Source: quora.com
Alkali metals are very reactive. Alkali metals are among the most reactive metals. Ionization energy is the energy required to remove one electron from the atom. So they readily react with water oxygen which are common constituents in the environment. This is due in part to their larger atomic radii and low ionization energies.
 Source: slideplayer.com
Source: slideplayer.com
Sodium and potassium are most common cations in biological systems. The alkali metals are soft metals that are highly reactive with water and oxygen. Alkali metals are in group 1 so they have only one electron in their outermost shell. They are trying to loose this electron so they can obtain a noble gas configuration. This means that they will react violently to do so as it does not require very much energy to pull away that one electron.
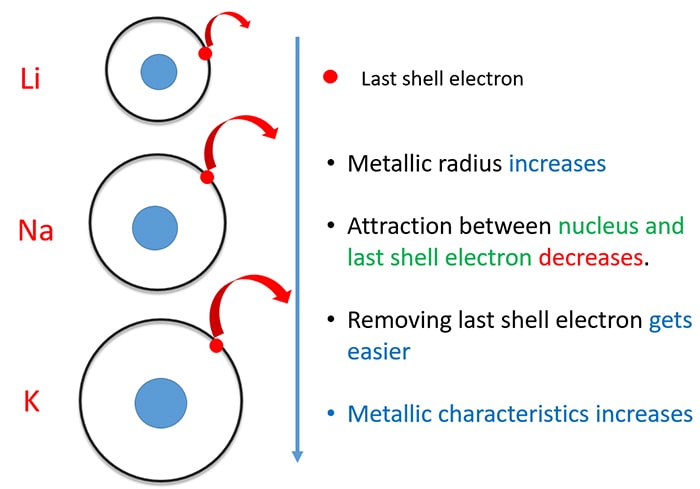 Source: chemistryscl.com
Source: chemistryscl.com
Biological importance of sodium and potassium. This is due in part to their larger atomic radii and low ionization energies. Alkali metals are in group 1 so they have only one electron in their outermost shell. These metals are characterized by their soft texture and silvery color. This means that they will react violently to do so as it does not require very much energy to pull away that one electron.
 Source: quora.com
Source: quora.com
These metals are characterized by their soft texture and silvery color. This is due in part to their larger atomic radii and low ionization energies. Biological importance of sodium and potassium. Alkali metals are among the most reactive metals. Alkali metals are in group 1 so they have only one electron in their outermost shell.
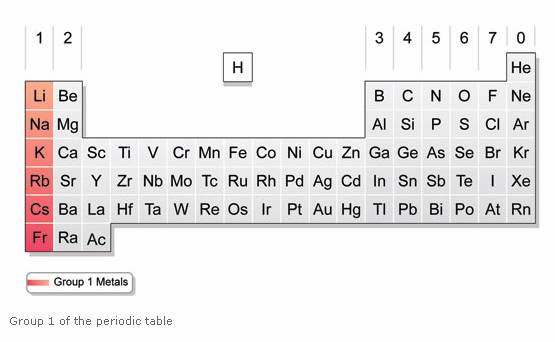 Source: onlinemathlearning.com
Source: onlinemathlearning.com
They are trying to loose this electron so they can obtain a noble gas configuration. So they readily react with water oxygen which are common constituents in the environment. They are trying to loose this electron so they can obtain a noble gas configuration. Alkali metals are in group 1 so they have only one electron in their outermost shell. They tend to donate their electrons in reactions and have an oxidation state of 1.
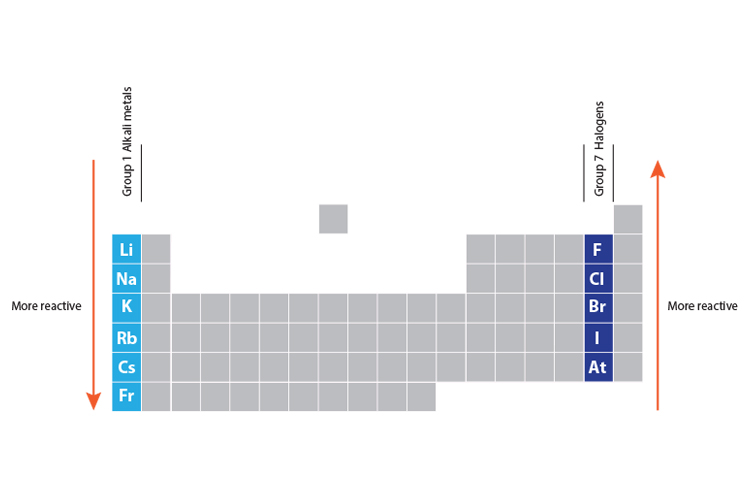 Source: mammothmemory.net
Source: mammothmemory.net
Alkali metals are in group 1 so they have only one electron in their outermost shell. Alkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. As a result of their weak electrostatic attraction metals have low ionisation energies meaning it s easier for them to lose electrons. Alkali metals are reactive because of their ionization energies.
 Source: slideplayer.com
Source: slideplayer.com
They tend to donate their electrons in reactions and have an oxidation state of 1. Alkali metals are reactive because of their ionization energies. This is due in part to their larger atomic radii and low ionization energies. They are trying to loose this electron so they can obtain a noble gas configuration. Sodium and potassium are most common cations in biological systems.
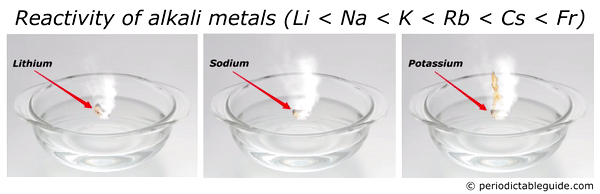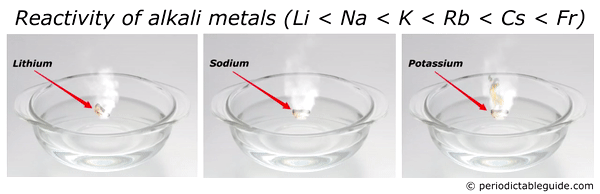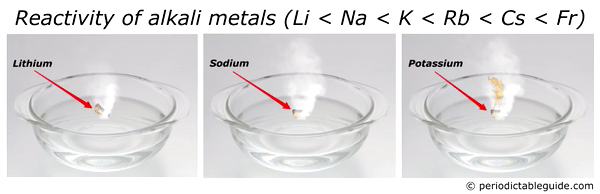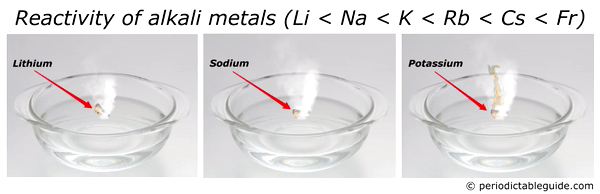 Source: periodictableguide.com
Source: periodictableguide.com
This means that they will react violently to do so as it does not require very much energy to pull away that one electron. As a result of their weak electrostatic attraction metals have low ionisation energies meaning it s easier for them to lose electrons. Alkali metals are reactive because of their ionization energies. They re so soft that you can cut them with a plastic knife. When it s easier for a metal to lose electrons that means.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why are alkali metals so reactive by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.